- Record: found
- Abstract: found
- Article: found
Clinical and pathogenic aspects of the severe cutaneous adverse reaction epidermal necrolysis (EN)
Read this article at
Abstract
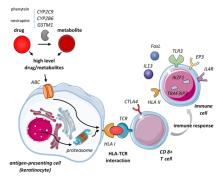
The severe cutaneous adverse reaction epidermal necrolysis (EN) which includes toxic epidermal necrolysis and the milder Stevens‐Johnson syndrome is characterized by epidermal loss due to massive keratinocyte apoptosis and/or necroptosis. EN is often caused by a drug mediating a specific TCR‐HLA interaction via the (pro)hapten, pharmacological interaction or altered peptide loading mechanism involving a self‐peptide presented by keratinocytes. (Memory) CD8 + T cells are activated and exhibit cytotoxicity against keratinocytes via the perforin/granzyme B and granulysin pathway and Fas/FasL interaction. Alternatively drug‐induced annexin release by CD14 + monocytes can induce formyl peptide receptor 1 death of keratinocytes by necroptosis. Subsequent keratinocyte death stimulates local inflammation, activating other immune cells producing pro‐inflammatory molecules and downregulating regulatory T cells. Widespread epidermal necrolysis and inflammation can induce life‐threatening systemic effects, leading to high mortality rates. Research into genetic susceptibility aims to identify risk factors for eventual prevention of EN. Specific HLA class I alleles show the strongest association with EN, but risk variants have also been identified in genes involved in drug metabolism, cellular drug uptake, peptide presentation and function of CD8 + T cells and other immune cells involved in cytotoxic responses. After the acute phase of EN, long‐term symptoms can remain or arise mainly affecting the skin and eyes. Mucosal sequelae are characterized by occlusions and strictures due to adherence of denuded surfaces and fibrosis following mucosal inflammation. In addition, systemic pathology can cause acute and chronic hepatic and renal symptoms. EN has a large psychological impact and strongly affects health‐related quality of life among EN survivors.
Related collections
Most cited references148
- Record: found
- Abstract: found
- Article: not found
SCORTEN: a severity-of-illness score for toxic epidermal necrolysis.
- Record: found
- Abstract: found
- Article: not found
Immune self-reactivity triggered by drug-modified HLA-peptide repertoire.
- Record: found
- Abstract: found
- Article: not found