- Record: found
- Abstract: found
- Article: found
Global and Ocular Hypothermic Preconditioning Protect the Rat Retina from Ischemic Damage
Read this article at
Abstract
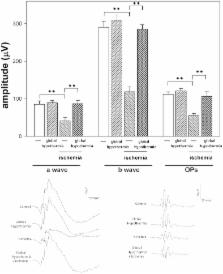
Retinal ischemia could provoke blindness. At present, there is no effective treatment against retinal ischemic damage . Strong evidence supports that glutamate is implicated in retinal ischemic damage. We investigated whether a brief period of global or ocular hypothermia applied 24 h before ischemia (i.e. hypothermic preconditioning, HPC) protects the retina from ischemia/reperfusion damage, and the involvement of glutamate in the retinal protection induced by HPC. For this purpose, ischemia was induced by increasing intraocular pressure to 120 mm Hg for 40 min. One day before ischemia, animals were submitted to global or ocular hypothermia (33°C and 32°C for 20 min, respectively) and fourteen days after ischemia, animals were subjected to electroretinography and histological analysis. Global or ocular HPC afforded significant functional (electroretinographic) protection in eyes exposed to ischemia/reperfusion injury. A marked alteration of the retinal structure and a decrease in retinal ganglion cell number were observed in ischemic retinas, whereas global or ocular HPC significantly preserved retinal structure and ganglion cell count. Three days after ischemia, a significant decrease in retinal glutamate uptake and glutamine synthetase activity was observed, whereas ocular HPC prevented the effect of ischemia on these parameters. The intravitreal injection of supraphysiological levels of glutamate induced alterations in retinal function and histology which were significantly prevented by ocular HPC. These results support that global or ocular HPC significantly protected retinal function and histology from ischemia/reperfusion injury, probably through a glutamate-dependent mechanism.
Related collections
Most cited references47
- Record: found
- Abstract: found
- Article: not found
Retinal ischemia: mechanisms of damage and potential therapeutic strategies.
- Record: found
- Abstract: found
- Article: not found
Small differences in intraischemic brain temperature critically determine the extent of ischemic neuronal injury.
- Record: found
- Abstract: found
- Article: not found