- Record: found
- Abstract: found
- Article: found
Targeted versus non-targeted HIV testing offered via electronic questionnaire in a Swiss emergency department: A randomized controlled study
Read this article at
Abstract
Background
In Switzerland, the national HIV testing recommendations propose targeted testing. Although the emergency department (ED) is mentioned specifically as a site where HIV testing should take place, the testing rate in our ED is 1% of patients seen. The aim of this study was to use electronic tablets to offer testing to ED patients and to examine whether non-targeted screening increased testing rates compared to targeted testing.
Methods
This randomised, cross-over design study took place at Lausanne University Hospital, Switzerland, between August and November 2015. Eligible patients were randomised to a targeted testing or a non-targeted screening arm. Using electronic tablets, targeted arm patients completed a risk factor assessment; patients with risk factors were offered free rapid HIV testing. Non-targeted arm patients received information about HIV and HIV testing on their tablet and were then offered testing. In a second step, patients who declined testing were crossed over to the other strategy. The primary endpoint was the HIV testing rate per arm.
Results
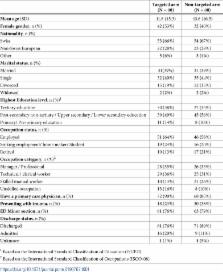
Eighty patients were recruited to each study arm. In the targeted arm, 17 patients (of 80, 21%) had at least one risk factor and were offered testing, of whom eight (of 17, 47%) accepted. HIV testing rate in the targeted arm was 10% (8/80) compared to 48% (38/80) in the non-targeted arm ( P<0.001). Secondary cross–screening, where targeted arm patients without risk factors were offered non-targeted screening, increased the testing rate in the targeted arm to 45% (36/80). Among patients offered testing, the acceptance rate did not differ between targeted and non-targeted arms, at 48% and 53%, respectively ( P = 0.9)
Discussion
In our centre, non-targeted HIV screening resulted in a higher testing rate than targeted testing due to more patients being offered a test. The acceptance rate of testing offered did not differ between targeted and non-targeted arms. Electronic tablets were well-received by patients and easy to use. We conclude that non-targeted HIV screening using electronic tablets would increase the HIV testing rate in our ED.
Related collections
Most cited references22
- Record: found
- Abstract: found
- Article: not found
Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health-care settings.
- Record: found
- Abstract: found
- Article: not found
Computer-delivered interventions for health promotion and behavioral risk reduction: a meta-analysis of 75 randomized controlled trials, 1988-2007.
- Record: found
- Abstract: found
- Article: not found