- Record: found
- Abstract: found
- Article: not found
Results from Screening Polyurethane Foam Based Consumer Products for Flame Retardant Chemicals: Assessing Impacts on the Change in the Furniture Flammability Standards
Read this article at
Abstract

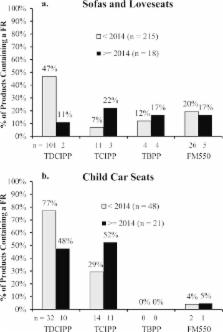
Flame retardant (FR) chemicals have often been added to polyurethane foam to meet required state and federal flammability standards. However, some FRs (e.g., PBDEs and TDCIPP) are associated with health hazards and are now restricted from use in some regions. In addition, California’s residential furniture flammability standard (TB-117) has undergone significant amendments over the past few years, and TDCIPP has been added to California’s Proposition 65 list. These events have likely led to shifts in the types of FRs used, and the products to which they are applied. To provide more information on the use of FRs in products containing polyurethane foam (PUF), we established a screening service for the general public. Participants residing in the US were allowed to submit up to 5 samples from their household for analysis, free of charge, and supplied information on the product category, labeling, and year and state of purchase. Between February 2014 and June 2016, we received 1141 PUF samples for analysis from various products including sofas, chairs, mattresses, car seats and pillows. Of these samples tested, 52% contained a FR at levels greater than 1% by weight. Tris(1,3-dichloroisopropyl)phosphate (TDCIPP) was the most common FR detected in PUF samples, and was the most common FR detected in all product categories. Analysis of the data by purchasing date suggests that the use of TDCIPP decreased in recent years, paralleled with an increase in the use of TCIPP and a nonhalogenated aryl phosphate mixture we call “TBPP.” In addition, we observed significant decreases in FR applications in furniture products and child car seats, suggesting the use of additive FRs in PUF may be declining, perhaps as a reflection of recent changes to TB-117 and Proposition 65. More studies are needed to determine how these changes in FR use relate to changes in exposure among the general population.
Related collections
Most cited references31
- Record: found
- Abstract: found
- Article: not found
Phosphorus flame retardants: properties, production, environmental occurrence, toxicity and analysis.
- Record: found
- Abstract: found
- Article: not found
Brominated flame retardants: cause for concern?
- Record: found
- Abstract: found
- Article: not found

