- Record: found
- Abstract: found
- Article: found
Accreditation program for gastrointestinal endoscopes reprocessing in Italy: An on-site survey
Read this article at
Abstract
Background and study aims Endoscope reprocessing has been associated with a variable failure rate. Our aim was to present an overview on current practices for reprocessing in Italian facilities and discuss the principle critical points.
Methods In 2014 the Italian Society for Digestive Diseases implemented an accreditation program in collaboration with an independent organization for certification and with the Italian Association for Endoscopy Technical Operators. During a 1-day site visit of the endoscopy center, two endoscopists, one nurse, and the representative of the certification body evaluated the endoscope reprocessing.
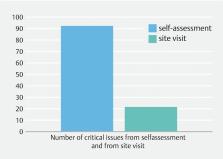
Results As of July 1, 2020, 28 endoscopy centers had been accredited. Ten centers are completing the measures to correct deficiencies found at the visit. Three centers withdrew from the program. The accreditation program has found variations between the various centers, confirming the poor compliance with guidelines. Major deviations from the standards, established by the model before the site visit according to national and international guidelines, concerned instrument cleaning (44.7 % of the centers), instrument storage (23.7 %), and microbiological tests (31.6 %).
Conclusions Our overview demonstrated the lack of many reprocessing phases, which are important to prevent endoscopy-associated infections. Accreditation can achieve a transformation in quality and safety of reprocessing with the Italian centrally-led approach.
Related collections
Most cited references24
- Record: found
- Abstract: found
- Article: not found
Reprocessing of flexible endoscopes and endoscopic accessories used in gastrointestinal endoscopy: Position Statement of the European Society of Gastrointestinal Endoscopy (ESGE) and European Society of Gastroenterology Nurses and Associates (ESGENA) – Update 2018
- Record: found
- Abstract: not found
- Article: not found
Multisociety guideline on reprocessing flexible GI endoscopes: 2016 update
- Record: found
- Abstract: found
- Article: not found