- Record: found
- Abstract: found
- Article: not found
Lgr5-Positive Supporting Cells Generate New Hair Cells in the Postnatal Cochlea

Read this article at
Summary
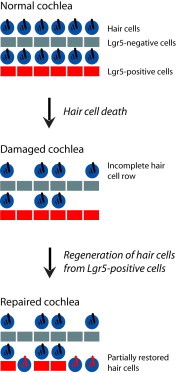
The prevalence of hearing loss after damage to the mammalian cochlea has been thought to be due to a lack of spontaneous regeneration of hair cells, the primary receptor cells for sound. Here, we show that supporting cells, which surround hair cells in the normal cochlear epithelium, differentiate into new hair cells in the neonatal mouse following ototoxic damage. Using lineage tracing, we show that new hair cells, predominantly outer hair cells, arise from Lgr5-expressing inner pillar and third Deiters cells and that new hair cell generation is increased by pharmacological inhibition of Notch. These data suggest that the neonatal mammalian cochlea has some capacity for hair cell regeneration following damage alone and that Lgr5-positive cells act as hair cell progenitors in the cochlea.
Graphical Abstract
Highlights
Abstract
Although hair cells, the primary receptor cells for sound, had been thought to be incapable of regeneration, Edge and colleagues find that supporting cells, which surround hair cells in the normal cochlear epithelium, include a subset of Lgr5-expressing cells that act as progenitor cells, differentiating into hair cells in the neonatal mouse after damage.
Related collections
Most cited references34
- Record: found
- Abstract: found
- Article: not found
Prestin is the motor protein of cochlear outer hair cells.
- Record: found
- Abstract: found
- Article: not found
Wnt signalling induces maturation of Paneth cells in intestinal crypts.
- Record: found
- Abstract: found
- Article: not found