- Record: found
- Abstract: found
- Article: found
Alterations of lipid metabolism provide serologic biomarkers for the detection of asymptomatic versus symptomatic COVID-19 patients
Read this article at
Abstract
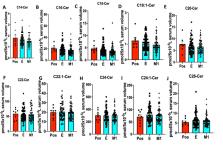
COVID-19 pandemic exerts a health care emergency around the world. The illness severity is heterogeneous. It is mostly unknown why some individuals who are positive for SARS-CoV-2 antibodies stay asymptomatic while others show moderate to severe disease symptoms. Reliable biomarkers for early detection of the disease are urgently needed to attenuate the virus’s spread and help make early treatment decisions. Bioactive sphingolipids play a crucial role in the regulation of viral infections and pro-inflammatory responses involved in the severity of COVID-19. However, any roles of sphingolipids in COVID-19 development or detection remain unknown. In this study, lipidomics measurement of serum sphingolipids demonstrated that reduced sphingosine levels are highly associated with the development of symptomatic COVID-19 in the majority (99.24%) SARS-CoV-2-infected patients compared to asymptomatic counterparts. The majority of asymptomatic individuals (73%) exhibited increased acid ceramidase (AC) in their serum, measured by Western blotting, consistent with elevated sphingosine levels compared to SARS-CoV-2 antibody negative controls. AC protein was also reduced in almost all of the symptomatic patients’ serum, linked to reduced sphingosine levels, measured in longitudinal acute or convalescent COVID-19 samples. Thus, reduced sphingosine levels provide a sensitive and selective serologic biomarker for the early identification of asymptomatic versus symptomatic COVID-19 patients.
Related collections
Most cited references61
- Record: found
- Abstract: found
- Article: found
Remdesivir for the Treatment of Covid-19 — Final Report
- Record: found
- Abstract: found
- Article: not found
Clinical and immunologic features in severe and moderate Coronavirus Disease 2019
- Record: found
- Abstract: found
- Article: not found