- Record: found
- Abstract: found
- Article: found
CD200 in CNS tumor-induced immunosuppression: the role for CD200 pathway blockade in targeted immunotherapy
Read this article at
Abstract
Background
Immunological quiescence in the central nervous system (CNS) is a potential barrier to immune mediated anti-tumor response. One suppressive mechanism results from the interaction of parenchyma-derived CD200 and its receptor on myeloid cells. We suggest that CD200/CD200R interactions on myeloid cells expand the myeloid-derived suppressor cell (MDSC) population and that blocking tumor-derived CD200 will enhance the efficacy of immunotherapy.
Methods
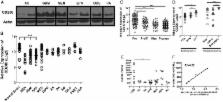
CD200 mRNA expression levels in human brain tumor tissue samples were measured by microarray. The amount of circulating CD200 protein in the sera of patients with brain tumors was determined by ELISA and, when corresponding peripheral blood samples were available, was correlated quantitatively with MDSCs. CD200-derived peptides were used as competitive inhibitors in a mouse model of glioblastoma immunotherapy.
Results
CD200 mRNA levels were measured in human brain tumors, with different expression levels being noted among the sub groups of glioblastoma, medulloblastoma and ependymoma. Serum CD200 concentrations were highest in patients with glioblastoma and correlated significantly with MDSC expansion. Similarly, in vitro studies determined that GL261 cells significantly expanded a MDSC population. Interestingly, a CD200R antagonist inhibited the expansion of murine MDSCs in vitro and in vivo. Moreover, inclusion of CD200R antagonist peptide in glioma tumor lysate-derived vaccines slowed tumor growth and significantly enhanced survival.
Conclusion
These data suggest that CNS-derived tumors can evade immune surveillance by engaging CD200. Because of the homology between mouse and human CD200, our data also suggest that blockade of CD200 binding to its receptor will enhance the efficacy of immune mediated anti-tumor strategies for brain tumors.
Related collections
Most cited references30
- Record: found
- Abstract: found
- Article: not found
PD-1 and CTLA-4 combination blockade expands infiltrating T cells and reduces regulatory T and myeloid cells within B16 melanoma tumors.
- Record: found
- Abstract: found
- Article: not found
Immunotherapy of cancer in 2012.
- Record: found
- Abstract: found
- Article: not found