- Record: found
- Abstract: found
- Article: found
Keratin-mediated hair growth and its underlying biological mechanism
Read this article at
Abstract
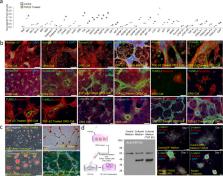
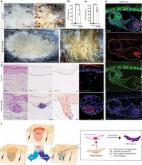
Here we show that intradermal injection of keratin promotes hair growth in mice, which results from extracellular interaction of keratin with hair forming cells. Extracellular application of keratin induces condensation of dermal papilla cells and the generation of a P-cadherin-expressing cell population (hair germ) from outer root sheath cells via keratin-mediated microenvironmental changes. Exogenous keratin-mediated hair growth is reflected by the finding that keratin exposure from transforming growth factor beta 2 (TGFβ2)-induced apoptotic outer root sheath cells appears to be critical for dermal papilla cell condensation and P-cadherin-expressing hair germ formation. Immunodepletion or downregulation of keratin released from or expressed in TGFβ2-induced apoptotic outer root sheath cells negatively influences dermal papilla cell condensation and hair germ formation. Our pilot study provides an evidence on initiating hair regeneration and insight into the biological function of keratin exposed from apoptotic epithelial cells in tissue regeneration and development.
Abstract
Injecting human hair-derived keratin into mice skin accelerates hair growth & formation, as TGFβ2 secretion during hair destruction stimulates epithelial cell death and keratin release, leading to dermal cell condensation & hair growth.
Related collections
Most cited references50
- Record: found
- Abstract: found
- Article: not found
Defining the epithelial stem cell niche in skin.
- Record: found
- Abstract: not found
- Article: not found
β-Catenin Controls Hair Follicle Morphogenesis and Stem Cell Differentiation in the Skin
- Record: found
- Abstract: found
- Article: found