- Record: found
- Abstract: found
- Article: found
MicroRNA‐130a regulates neurological deficit and angiogenesis in rats with ischaemic stroke by targeting XIAP
Read this article at
Abstract
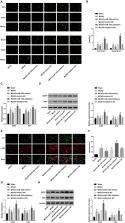
MicroRNAs (miRNAs) have already been proposed to be implicated in the development of ischaemic stroke. We aim to investigate the role of miR‐130a in the neurological deficit and angiogenesis in rats with ischaemic stroke by regulating X‐linked inhibitor of apoptosis protein (XIAP). Middle cerebral artery occlusion (MCAO) models were established by suture‐occluded method, and MCAO rats were then treated with miR‐130a mimics/inhibitors or/and altered XIAP for detection of changes of rats’ neurological function, nerve damage and angiogenesis in MCAO rats. The oxygen‐glucose deprivation (OGD) cellular models were established and respectively treated to determine the roles of miR‐130a and XIAP in neuronal viability and apoptosis. The expression levels of miR‐130a and XIAP in brain tissues of MCAO rats and OGD‐treated neurons were detected. The binding site between miR‐130a and XIAP was verified by luciferase activity assay. MiR‐130a was overexpressed while XIAP was down‐regulated in MCAO rats and OGD‐treated neurons. In animal models, suppressed miR‐130a improved neurological function, alleviated nerve damage and increased new vessels in brain tissues of rats with MCAO. In cellular models, miR‐130a inhibition promoted neuronal viability and suppressed apoptosis. Inhibited XIAP reversed the effect of inhibited miR‐130a in both MCAO rats and OGD‐treated neurons. XIAP was identified as a target of miR‐130a. Our study reveals that miR‐130a regulates neurological deficit and angiogenesis in rats with MCAO by targeting XIAP.
Related collections
Most cited references32
- Record: found
- Abstract: found
- Article: not found
MiR-200, a new star miRNA in human cancer.
- Record: found
- Abstract: found
- Article: not found
XIAP inhibits caspase-3 and -7 using two binding sites: evolutionarily conserved mechanism of IAPs.
- Record: found
- Abstract: found
- Article: not found