- Record: found
- Abstract: found
- Article: found
MicroRNA-378 Regulates Adiponectin Expression in Adipose Tissue: A New Plausible Mechanism
Read this article at
Abstract
Aims
Mechanisms regulating adiponectin expression have not been fully clarified. MicroRNAs (miRNAs), small non-coding RNAs that regulate gene expression, are involved in biological processes, including obesity and insulin resistance. We evaluated whether the miRNA-378 pathway is involved in regulating adiponectin expression.
Methods and Results
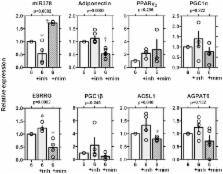
First, we determined a putative target site for miRNA-378 in the 3 prime untranslated region (3'UTR) of the adiponectin gene by in silico analysis. The levels of adiponectin mRNA and protein were decreased in 3T3-L1 cells overexpressing the mimic of miRNA-378. Luminescence activity in HEK293T cells expressing a renilla-luciferase-adiponectin-3'UTR sequence was inhibited by overexpressing the mimic of miRNA-378, and the decrease was reversed by adding the inhibitor of miRNA-378. Moreover, we confirmed the inhibitory effects of the mimic were cancelled in a deleted mutant of the miR-378 3′-UTR binding site. Addition of tumor necrosis factor-α (TNF α) led a upregulation of miR-378 and downregulation of adiponectin at mRNA and protein levels in 3T3-L1 cells. Level of miR-378 was higher and mRNA level of adiponectin was lower in diabetic ob/ob mice than those of normal C57BL/6 mice and levels of miR378 and adiponectin were negatively well correlated (r = −0.624, p = 0.004).
Related collections
Most cited references14
- Record: found
- Abstract: found
- Article: not found
Control of mitochondrial metabolism and systemic energy homeostasis by microRNAs 378 and 378*.
- Record: found
- Abstract: found
- Article: not found
Therapy Insight: adipocytokines in metabolic syndrome and related cardiovascular disease.
- Record: found
- Abstract: found
- Article: not found