- Record: found
- Abstract: found
- Article: found
Cyclic-di-GMP signalling and biofilm-related properties of the Shiga toxin-producing 2011 German outbreak Escherichia coli O104:H4
Read this article at
Abstract
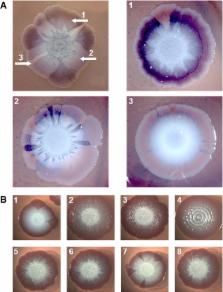
In 2011, nearly 4,000 people in Germany were infected by Shiga toxin (Stx)-producing Escherichia coli O104:H4 with > 22% of patients developing haemolytic uraemic syndrome (HUS). Genome sequencing showed the outbreak strain to be related to enteroaggregative E. coli (EAEC), suggesting its high virulence results from EAEC-typical strong adherence and biofilm formation combined to Stx production. Here, we report that the outbreak strain contains a novel diguanylate cyclase (DgcX)—producing the biofilm-promoting second messenger c-di-GMP—that shows higher expression than any other known E. coli diguanylate cyclase. Unlike closely related E. coli, the outbreak strain expresses the c-di-GMP-controlled biofilm regulator CsgD and amyloid curli fibres at 37°C, but is cellulose-negative. Moreover, it constantly generates derivatives with further increased and deregulated production of CsgD and curli. Since curli fibres are strongly proinflammatory, with cellulose counteracting this effect, high c-di-GMP and curli production by the outbreak O104:H4 strain may enhance not only adherence but may also contribute to inflammation, thereby facilitating entry of Stx into the bloodstream and to the kidneys where Stx causes HUS.
Related collections
Most cited references77
- Record: found
- Abstract: found
- Article: not found
SMART, a simple modular architecture research tool: identification of signaling domains.
- Record: found
- Abstract: found
- Article: not found
German outbreak of Escherichia coli O104:H4 associated with sprouts.
- Record: found
- Abstract: found
- Article: not found