- Record: found
- Abstract: found
- Article: found
Direct provision versus facility collection of HIV self-tests among female sex workers in Uganda: A cluster-randomized controlled health systems trial
Read this article at
Abstract
Background
HIV self-testing allows HIV testing at any place and time and without health workers. HIV self-testing may thus be particularly useful for female sex workers (FSWs), who should test frequently but face stigma and financial and time barriers when accessing healthcare facilities.
Methods and findings
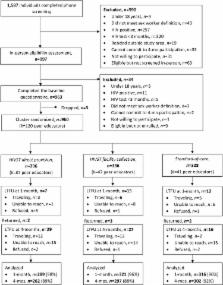
We conducted a cluster-randomized controlled health systems trial among FSWs in Kampala, Uganda, to measure the effect of 2 HIV self-testing delivery models on HIV testing and linkage to care outcomes. FSW peer educator groups (1 peer educator and 8 participants) were randomized to either (1) direct provision of HIV self-tests, (2) provision of coupons for free collection of HIV self-tests in a healthcare facility, or (3) standard of care HIV testing. We randomized 960 participants in 120 peer educator groups from October 18, 2016, to November 16, 2016. Participants’ median age was 28 years (IQR 24–32). Our prespecified primary outcomes were self-report of any HIV testing at 1 month and at 4 months; our prespecified secondary outcomes were self-report of HIV self-test use, seeking HIV-related medical care and ART initiation. In addition, we analyzed 2 secondary outcomes that were not prespecified: self-report of repeat HIV testing—to understand the intervention effects on frequent testing—and self-reported facility-based testing—to quantify substitution effects. Participants in the direct provision arm were significantly more likely to have tested for HIV than those in the standard of care arm, both at 1 month (risk ratio [RR] 1.33, 95% CI 1.17–1.51, p < 0.001) and at 4 months (RR 1.14, 95% CI 1.07–1.22, p < 0.001). Participants in the direct provision arm were also significantly more likely to have tested for HIV than those in the facility collection arm, both at 1 month (RR 1.18, 95% CI 1.07–1.31, p = 0.001) and at 4 months (RR 1.03, 95% CI 1.01–1.05, p = 0.02). At 1 month, fewer participants in the intervention arms had sought medical care for HIV than in the standard of care arm, but these differences were not significant and were reduced in magnitude at 4 months. There were no statistically significant differences in ART initiation across study arms. At 4 months, participants in the direct provision arm were significantly more likely to have tested twice for HIV than those in the standard of care arm (RR 1.51, 95% CI 1.29–1.77, p < 0.001) and those in the facility collection arm (RR 1.22, 95% CI 1.08–1.37, p = 0.001). Participants in the HIV self-testing arms almost completely replaced facility-based testing with self-testing. Two adverse events related to HIV self-testing were reported: interpersonal violence and mental distress. Study limitations included self-reported outcomes and limited generalizability beyond FSWs in similar settings.
Conclusions
In this study, HIV self-testing appeared to be safe and increased recent and repeat HIV testing among FSWs. We found that direct provision of HIV self-tests was significantly more effective in increasing HIV testing among FSWs than passively offering HIV self-tests for collection in healthcare facilities. HIV self-testing could play an important role in supporting HIV interventions that require frequent HIV testing, such as HIV treatment as prevention, behavior change for transmission reduction, and pre-exposure prophylaxis.
Abstract
In a cluster-randomized trial, Katrina Ortblad and colleagues study the provision of HIV self-tests for female sex workers in Uganda.
Author summary
Why was this study done?
-
HIV self-testing can increase the convenience and privacy of HIV testing because it allows individuals to test for HIV at any time and place they choose and without the presence of another person.
-
Many sub-Saharan governments are considering HIV self-testing for FSWs, but little evidence exists on the delivery, uptake, and effects of HIV self-testing among members of this population.
What did the researchers do and find?
-
We randomized 960 FSWs in 120 peer educator groups 1:1:1 to (1) direct provision of HIV self-tests, (2) provision of a coupon for free collection of HIV self-tests in a healthcare facility, and (3) standard of care facility-based HIV testing services. Participants received 1 HIV self-test (in the direct provision arm) or 1 coupon (in the facility collection arm) shortly after randomization and again 3 months later.
-
Any recent HIV testing (at 1 month and at 4 months) and repeat HIV testing (at 4 months) was significantly greater in the direct provision arm compared to the facility collection arm and the standard of care arm.
-
FSWs in the intervention arms almost completely substituted HIV self-testing for facility-based HIV testing.
-
Fewer FSWs in the interventions arms sought HIV-related medical care than those in the standard of care arm at 1 month; however, this difference disappeared by 4 months.
What do these findings mean?
-
HIV self-testing appears to be safe and increases recent and repeat HIV testing among FSW in Uganda.
-
The active approach to directly provide HIV self-tests to FSWs is more effective in increasing HIV testing than the passive approach to offer free access to HIV self-tests via coupons that can be used to collect self-tests in healthcare facilities.
-
Almost all FSWs who had access to HIV self-testing used this new testing technology instead of facility-based HIV testing.
-
It is possible that self-testing in homes and other private locations (rather than in healthcare facilities) may reduce linkage to care in the short term.
-
Sub-Saharan African governments should consider HIV self-testing for FSWs but ensure it is delivered with linkage-enhancing interventions. Where possible, governments should introduce active HIV self-testing models (delivering HIV self-tests directly to FSWs) rather than passive models (requiring FSWs to collect self-tests in healthcare facilities).
Related collections
Most cited references21
- Record: found
- Abstract: not found
- Article: not found
Intention-to-treat principle.
- Record: found
- Abstract: found
- Article: not found
Accuracy and Acceptability of Oral Fluid HIV Self-Testing in a General Adult Population in Kenya
- Record: found
- Abstract: found
- Article: not found