- Record: found
- Abstract: found
- Article: found
Acetic Acid Ketonization over Fe 3O 4/SiO 2 for Pyrolysis Bio‐Oil Upgrading
Read this article at
Abstract
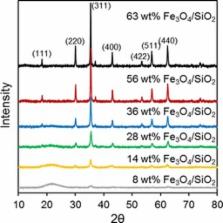
A family of silica‐supported, magnetite nanoparticle catalysts was synthesised and investigated for continuous‐flow acetic acid ketonisation as a model pyrolysis bio‐oil upgrading reaction. The physico‐chemical properties of Fe 3O 4/SiO 2 catalysts were characterised by using high‐resolution transmission electron microscopy, X‐ray absorption spectroscopy, X‐ray photo‐electron spectroscopy, diffuse reflectance infrared Fourier transform spectroscopy, thermogravimetric analysis and porosimetry. The acid site densities were inversely proportional to the Fe 3O 4 particle size, although the acid strength and Lewis character were size‐invariant, and correlated with the specific activity for the vapour‐phase acetic ketonisation to acetone. A constant activation energy (∼110 kJ mol −1), turnover frequency (∼13 h −1) and selectivity to acetone of 60 % were observed for ketonisation across the catalyst series, which implies that Fe 3O 4 is the principal active component of Red Mud waste.
Related collections
Most cited references74
- Record: found
- Abstract: found
- Article: not found
Morphology and surface properties of fumed silicas.
- Record: found
- Abstract: not found
- Article: not found