- Record: found
- Abstract: found
- Article: found
Comparison of 99mTc radiolabeled somatostatin antagonist with [ 68 Ga]Ga-DOTA-TATE in a patient with advanced neuroendocrine tumor
brief-report
Marta Opalinska
1 ,
Luka Lezaic
2
,
3 ,
Clemens Decristoforo
4 ,
Petra Kolenc
2
,
5 ,
Renata Mikolajczak
6 ,
Andrej Studen
7
,
8 ,
Urban Simoncic
7
,
8 ,
Irene Virgolini
4 ,
Malgorzata Trofimiuk-Muldner
1 ,
Piotr Garnuszek
6 ,
Christine Rangger
4 ,
Melpomeni Fani
9 ,
Boguslaw Glowa
10 ,
Konrad Skorkiewicz
10 ,
Alicja Hubalewska-Dydejczyk
1
,
15 July 2023
Read this article at
There is no author summary for this article yet. Authors can add summaries to their articles on ScienceOpen to make them more accessible to a non-specialist audience.
Abstract
Gallium-68-labelled somatostatin analogues are a gold standard of neuroendocrine tumors
(NETs) PET imaging being a tool for personalized treatment. However, in some cases
of low somatostatin receptor (SSTR) expressing tumors, its clinical value can be limited.
SSTR antagonists in comparison to currently used agonistic analogues recognize more
binding sites on NET cells [1], which may improve the diagnostic efficacy enabling
more precise staging leading to the better outcome.
SPECT radiopharmaceuticals represent the cornerstone of molecular imaging due to their
wide availability [2] and the development of a radiopharmaceutical based on technetium-99 m-labeled
SSTR antagonist would improve access to such clinically feasible diagnostic tool.
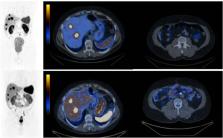
Below (Fig. 1), we present a comparison of SPECT/CT with a SSTR antagonist [99mTc]Tc-TECANT1
(N4-p-Cl-Phe-cyclo(D-Cys-Tyr-D-Aph(Cbm)-Lys-Thr-Cys)-D-Tyr-NH2, where D-Aph(Cbm):
D-4-amino-carbamoyl-phenylalanine) [3] and [68 Ga]Ga-DOTA-TATE PET/CT (EudraCT no
2019–003379-20). Please note better visualization of the lesions seen in the study
with [99mTc]Tc-TECANT1 (in 6 out of 7 lesions) as well as significantly higher tumor-to-background
ratio (in primary and metastatic lesions) obtained with the novel tracer (measured
as an absolute number of counts in lesion to background (TBR)) in comparison to [68 Ga]Ga-DOTA-TATE
PET/CT (4,07 (range 1,36–7,58) vs 2,26 (range 1,15–3,39)).
Fig. 1 Maximum intensity projection (MIP) and axial fused images: upper line -[99mTc]Tc-TECANT1
SPECT/CT (4 h post injection, injected activity 785 MBq, 120 frames, 20 s per frame);
bottom line — [68 Ga]Ga-DOTA-TATE PET/CT (1 h post injection, injected activity 146 MBq;
3 min per bed). Scans were obtained within 13 days. Long-acting somatostatin analogue
was withdrawn 4 weeks before imaging
Although PET is considered more sensitive than SPECT [4], the presented new radiopharmaceutical
holds promise to provide higher TBR values compared to the current gold standard 68 Ga-DOTA-TATE
PET/CT. In combination with the development of quantitative SPECT imaging of NETs,
the use of 99mTc-labelled SSTR antagonists could provide a widely available, clinically
significant step in the personalized management of NETs.
Related collections
Most cited references4
- Record: found
- Abstract: found
- Article: not found
Somatostatin Receptor Antagonists for Imaging and Therapy.
Damian Wild, Melpomeni Fani (2017)
- Record: found
- Abstract: found
- Article: not found