- Record: found
- Abstract: found
- Article: found
CAPTEM in Metastatic Well-Differentiated Intermediate to High Grade Neuroendocrine Tumors: A Single Centre Experience
Read this article at
Abstract
Introduction
Capecitabine-temozolomide (CAPTEM) has significant activity in patients (pts) with metastatic low grade pancreatic neuroendocrine tumors (NETs). However, there is limited data regarding its activity in pts with metastatic well-differentiated intermediate and high grade pancreatic and nonpancreatic NETs. The objective of this study was to assess the functional imaging response, survival, and tolerability of CAPTEM in this population.
Methods
A retrospective audit of pts with metastatic well-differentiated intermediate (WHO grade 2) or high grade (WHO grade 3) NETs treated at Peter MacCallum Cancer Centre between March 2013 and March 2017. Pts received capecitabine 750 mg/m 2 orally twice daily (bd) from days1 to 14 and temozolomide 100 mg/m 2 bd from days 10 to 14 every 28 days. Data regarding functional imaging response, progression-free and overall survival, and toxicities was collected.
Results
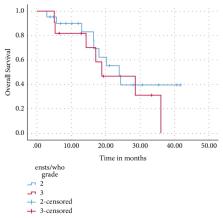
Thirty-two pts received a median of 6 cycles (range: 2-16) of CAPTEM for grade 2 (n=21, 66%) or grade 3 (n=11, 34%), Ki67 <55% (n= 7, 21.9%) or Ki67 ≥55% (n= 4, 12.5 %) NET. Primary site included gastroenteropancreatic (n= 17, 53%), lung (n= 12, 37.5%), and unknown origin (n = 3, 9.4%). Twenty-two percent received CAPTEM as first-line therapy. After a median of 31 months of follow-up, the two-year overall survival (OS) was 42%, with a median OS of 24 months. There was a trend towards improved median progression-free survival (PFS) in pts with low grade 3 (Ki67<55%) versus high grade 3 (Ki67 ≥55%) NETs (15 vs 4 months, p= 0.11). Ten (31.3%) experienced grade 3/4 toxicity, with nausea (15.6%), thrombocytopaenia (12.5%), and fatigue (9.4%) the most common toxicities reported.
Related collections
Most cited references34
- Record: found
- Abstract: found
- Article: not found
From RECIST to PERCIST: Evolving Considerations for PET response criteria in solid tumors.
- Record: found
- Abstract: found
- Article: not found
Predictive and prognostic factors for treatment and survival in 305 patients with advanced gastrointestinal neuroendocrine carcinoma (WHO G3): the NORDIC NEC study.
- Record: found
- Abstract: found
- Article: found