- Record: found
- Abstract: found
- Article: found
225Ac-PSMA-617 in chemotherapy-naive patients with advanced prostate cancer: a pilot study
Read this article at
Abstract
Background
A remarkable therapeutic efficacy has been demonstrated with 225Ac-prostate-specific membrane antigen (PSMA)-617 in heavily pre-treated metastatic castration-resistant prostate cancer (mCRPC) patients. We report our experience with 225Ac-PSMA-617 therapy in chemotherapy-naïve patients with advanced metastatic prostate carcinoma.
Methods
Seventeen patients with advanced prostate cancer were selected for treatment with 225Ac-PSMA-617 in 2-month intervals, with initial activity of 8 MBq, then de-escalation to 7 MBq, 6 MBq or 4 MBq in cases of good response. In one patient, activity was escalated to 13 MBq in the third cycle. Fourteen patients had three treatment cycles administered, while in three patients treatment was discontinued after two cycles due to good response. Six out of 17 patients received additional treatments after the third cycle. Prostate-specific antigen (PSA) was measured every 4 weeks for PSA response assessment. 68Ga-PSMA-PET/CT was used for functional response assessment before each subsequent treatment cycle. Serial full blood count, renal function test, and liver function were obtained to determine treatment-related side effects.
Results
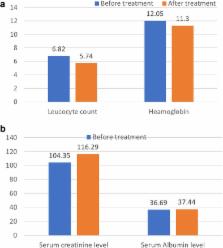
Good antitumor activity assessed by serum PSA level and 68Ga-PSMA-PET/CT was seen in 16/17 patients. In 14/17 patients, PSA decline ≥90% was seen after treatment, including seven patients with undetectable serum PSA following two (2/7) or three cycles (5/7) cycles of 225Ac-PSMA-617. Fifteen of 17 patients had a > 50% decline in lesions avidity for tracer on 68Ga-PSMA-PET/CT including 11 patients with complete resolution (PET-negative and either stable sclerosis on CT for bone or resolution of lymph node metastases) of all metastatic lesions. Grade 1/2 xerostomia was seen in all patients, and none was severe enough to lead to discontinuation of treatment. One patient had with extensive bone marrow metastases and a background anemia developed a grade 3 anemia while another patient with solitary kidney and pre-treatment grade 3 renal failure developed grade 4 renal toxicity following treatment. The group presented with significant palliation of bone pain and reduced toxicity to salivary glands due to de-escalation.
Conclusions
225Ac-PSMA-617 RLT of chemotherapy-naïve patients with advanced metastatic prostate carcinoma led to a ≥ 90% decline in serum PSA in 82% of patients including 41% of patients with undetectable serum PSA who remained in remission 12 months after therapy. The remarkable therapeutic efficacy reported in this study could be achieved with reduced toxicity to salivary glands due to de-escalation of administered activities in subsequent treatment cycles. This necessitates further exploration for informing clinical practice and clinical trial design.
Related collections
Most cited references17
- Record: found
- Abstract: found
- Article: not found
Targeted α-Therapy of Metastatic Castration-Resistant Prostate Cancer with225Ac-PSMA-617: Swimmer-Plot Analysis Suggests Efficacy Regarding Duration of Tumor Control

- Record: found
- Abstract: found
- Article: found
An Overview of Targeted Alpha Therapy with 225 Actinium and 213 Bismuth

- Record: found
- Abstract: found
- Article: found