- Record: found
- Abstract: found
- Article: found
Managing the Oocyte Meiotic Arrest—Lessons from Frogs and Jellyfish
Read this article at
Abstract
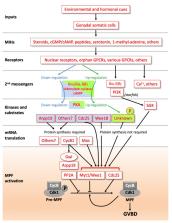
During oocyte development, meiosis arrests in prophase of the first division for a remarkably prolonged period firstly during oocyte growth, and then when awaiting the appropriate hormonal signals for egg release. This prophase arrest is finally unlocked when locally produced maturation initiation hormones (MIHs) trigger entry into M-phase. Here, we assess the current knowledge of the successive cellular and molecular mechanisms responsible for keeping meiotic progression on hold. We focus on two model organisms, the amphibian Xenopus laevis, and the hydrozoan jellyfish Clytia hemisphaerica. Conserved mechanisms govern the initial meiotic programme of the oocyte prior to oocyte growth and also, much later, the onset of mitotic divisions, via activation of two key kinase systems: Cdk1-Cyclin B/Gwl (MPF) for M-phase activation and Mos-MAPkinase to orchestrate polar body formation and cytostatic (CSF) arrest. In contrast, maintenance of the prophase state of the fully-grown oocyte is assured by highly specific mechanisms, reflecting enormous variation between species in MIHs, MIH receptors and their immediate downstream signalling response. Convergence of multiple signalling pathway components to promote MPF activation in some oocytes, including Xenopus, is likely a heritage of the complex evolutionary history of spawning regulation, but also helps ensure a robust and reliable mechanism for gamete production.
Related collections
Most cited references232
- Record: found
- Abstract: found
- Article: not found
Mechanisms of germ cell specification across the metazoans: epigenesis and preformation.
- Record: found
- Abstract: found
- Article: not found
Greatwall phosphorylates an inhibitor of protein phosphatase 2A that is essential for mitosis.
- Record: found
- Abstract: found
- Article: not found
