- Record: found
- Abstract: found
- Article: found
Relationship of the standard uptake value of 18F-FDG-PET-CT with tumor-infiltrating lymphocytes in breast tumors measuring ≥ 1 cm
Read this article at
Abstract
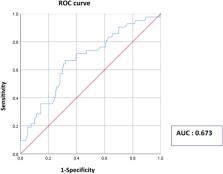
Evidence suggests that tumor cells and tumor-infiltrating lymphocytes (TILs) compete for glucose in the tumor microenvironment and that tumor metabolic parameters correlate with localized immune markers in several solid tumors. We investigated the relationship of the standardized uptake value (SUV) of 18F-fluorodeoxyglucose positron emission tomography computed tomography ( 18F-FDG-PET-CT) with stromal TIL levels in breast cancer. We included 202 patients who underwent preoperative 18F-FDG-PET-CT and had a tumor measuring ≥ 1 cm. Maximum SUV (SUVmax) was determined using 18F-FDG-PET-CT. Multiple logistic regression was used to identify factors related to high TIL levels (≥ 40%). All tumors were treatment naïve. A significant and weak correlation existed between continuous SUVmax and continuous TIL levels (p = 0.002, R = 0.215). Tumors with high SUVmax (≥ 4) had higher mean TIL levels than those with low SUVmax (< 4). In multivariable analysis, continuous SUVmax was an independent factor associated with high TIL levels; each 1-unit increment in SUVmax corresponded to an odds ratio of 1.14 (95% confidence interval: 1.01–1.29) for high TIL levels. Our study implies that SUV is associated with TILs in breast cancer and provides clinical evidence that elevated glucose uptake by breast tumors can predict the immune system-activated tumor micromilieu.
Related collections
Most cited references39
- Record: found
- Abstract: found
- Article: not found
TGF-β attenuates tumour response to PD-L1 blockade by contributing to exclusion of T cells
- Record: found
- Abstract: found
- Article: not found
Atezolizumab and Nab-Paclitaxel in Advanced Triple-Negative Breast Cancer
- Record: found
- Abstract: found
- Article: not found