- Record: found
- Abstract: found
- Article: found
An analysis of reported cases shoulder injury related to vaccine administration of after COVID-19 vaccination
Read this article at
ABSTRACT
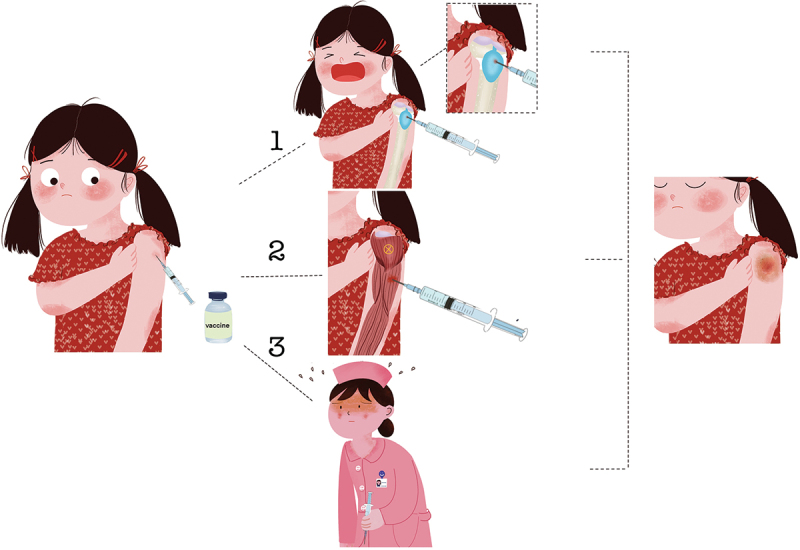
To prevent COVID-19, the COVID-19 vaccine has been widely administered worldwide, but various complications accompany this vaccine. The aim of this study was to investigate the demographic patterns, clinical features, diagnostic findings, and treatment outcomes associated with shoulder injury related to vaccine administration (SIRVA). This study examined 22 patients with SIRVA following COVID-19 vaccination from the Web of Science (WOS) and PubMed databases. The patients were categorized based on sex, age, type of COVID-19 vaccine received, dose administered, latency of symptom onset, and the presence of specific clinical manifestations. Patients, evenly distributed by sex (12 females, 10 males), and aged 21 to 84 years (mean age 46.6), were analyzed. SIRVA cases were reported across all age groups. The Pfizer – BioNTech COVID-19 vaccine had the highest incidence ( n = 8), followed by the Oxford/AstraZeneca COVID-19 vaccine ( n = 4). Symptoms, primarily shoulder pain ( n = 22) and shoulder mobility disorders ( n = 18), occurred within three days post-vaccination. Some patients also reported shoulder swelling ( n = 5) and fever ( n = 2). Imaging revealed nonspecific X-ray findings, supraspinatus tendon calcification ( n = 2), and shoulder edema and inflammation on MRI ( n = 12). This study provides insights into the clinical aspects of SIRVA related to COVID-19 vaccination. Recognition and appropriate management of these complications are crucial for optimal patient outcomes.
GRAPHICAL ABSTRACT
Related collections
Most cited references26
- Record: found
- Abstract: found
- Article: not found
Shoulder injury related to vaccine administration (SIRVA).
- Record: found
- Abstract: found
- Article: not found
Subacromial-subdeltoid bursitis following COVID-19 vaccination: a case of shoulder injury related to vaccine administration (SIRVA)
- Record: found
- Abstract: found
- Article: not found