- Record: found
- Abstract: found
- Article: found
Cerebral Autoregulation in Subarachnoid Hemorrhage
Read this article at
Abstract
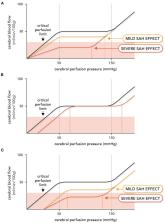
Subarachnoid hemorrhage (SAH) is a devastating stroke subtype with a high rate of mortality and morbidity. The poor clinical outcome can be attributed to the biphasic course of the disease: even if the patient survives the initial bleeding emergency, delayed cerebral ischemia (DCI) frequently follows within 2 weeks time and levies additional serious brain injury. Current therapeutic interventions do not specifically target the microvascular dysfunction underlying the ischemic event and as a consequence, provide only modest improvement in clinical outcome. SAH perturbs an extensive number of microvascular processes, including the “automated” control of cerebral perfusion, termed “ cerebral autoregulation.” Recent evidence suggests that disrupted cerebral autoregulation is an important aspect of SAH-induced brain injury. This review presents the key clinical aspects of cerebral autoregulation and its disruption in SAH: it provides a mechanistic overview of cerebral autoregulation, describes current clinical methods for measuring autoregulation in SAH patients and reviews current and emerging therapeutic options for SAH patients. Recent advancements should fuel optimism that microvascular dysfunction and cerebral autoregulation can be rectified in SAH patients.
Related collections
Most cited references318
- Record: found
- Abstract: found
- Article: not found
Guidelines for the management of aneurysmal subarachnoid hemorrhage: a guideline for healthcare professionals from the American Heart Association/american Stroke Association.
- Record: found
- Abstract: found
- Article: not found
Neurovascular coupling in the normal brain and in hypertension, stroke, and Alzheimer disease.
- Record: found
- Abstract: found
- Article: not found