- Record: found
- Abstract: found
- Article: found
Evidence for genetic association between chromosome 1q loci and predisposition to colorectal neoplasia
Read this article at
Abstract
Background:
A substantial fraction of familial colorectal cancer (CRC) and polyposis heritability remains unexplained. This study aimed to identify predisposing loci in patients with these disorders.
Methods:
Homozygosity mapping was performed using 222 563 SNPs in 302 index patients with various colorectal neoplasms and 3367 controls. Linkage analysis, exome and whole-genome sequencing were performed in a family affected by microsatellite stable CRCs. Candidate variants were genotyped in 10 554 cases and 21 480 controls. Gene expression was assessed at the mRNA and protein level.
Results:
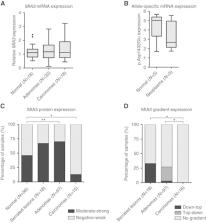
Homozygosity mapping revealed a disease-associated region at 1q32.3 which was part of the linkage region 1q32.2–42.2 identified in the CRC family. This includes a region previously associated with risk of CRC. Sequencing identified the p.Asp1432Glu variant in the MIA3 gene (known as TANGO1 or TANGO) and 472 additional rare, shared variants within the linkage region. In both cases and controls the population frequency was 0.02% for this MIA3 variant. The MIA3 mutant allele showed predominant mRNA expression in normal, cancer and precancerous tissues. Furthermore, immunohistochemistry revealed increased expression of MIA3 in adenomatous tissues.
Related collections
Most cited references60
- Record: found
- Abstract: found
- Article: not found
Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal.
- Record: found
- Abstract: found
- Article: not found
The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data.
- Record: found
- Abstract: found
- Article: not found