- Record: found
- Abstract: found
- Article: found
Thrombocytopenia in the ICU: disseminated intravascular coagulation and thrombotic microangiopathies—what intensivists need to know
editorial
Jean-Louis Vincent
1
,
,
Pedro Castro
2 ,
Beverley J. Hunt
3 ,
Achim Jörres
4 ,
Manuel Praga
5 ,
Jose Rojas-Suarez
6 ,
Eizo Watanabe
7
13 June 2018
Read this article at
There is no author summary for this article yet. Authors can add summaries to their articles on ScienceOpen to make them more accessible to a non-specialist audience.
Abstract
Thrombocytopenia affects 25–55% of intensive care unit (ICU) patients [1]. The reasons
for thrombocytopenia in the ICU are numerous, including, among others, sepsis, drugs,
and the use of extracorporeal devices (Fig. 1) [1]. Some patients with thrombocytopenia
also have microangiopathic hemolytic anemia (MAHA), accompanied by elevated serum
lactate dehydrogenase levels and schistocytes on the blood film [2, 3]. This combination
of thrombocytopenia and MAHA, in which thrombi form in the microvasculature and schistocytes
develop from red cell destruction as they pass over these thrombi [2], occurs in patients
with disseminated intravascular coagulation (DIC), but also in those with thrombotic
microangiopathies (TMAs), including thrombotic thrombocytopenic purpura (TTP) and
hemolytic uremic syndrome (HUS).
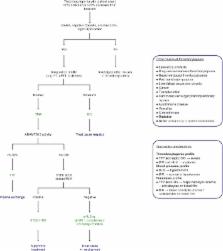
Fig. 1
An algorithm to rapidly differentiate disseminated intravascular coagulation (DIC)
from thrombotic thrombocytopenic purpura (TTP) and hemolytic uremic syndrome (HUS)
in the intensive care unit (ICU). Thrombocytopenia with microangiopathic hemolytic
anemia (MAHA), negative Coombs test, elevated lactate dehydrogenase (LDH), and organ
dysfunction are common to DIC, TTP, and HUS. Abnormal coagulation studies, including
prothrombin time (PT), activated partial thromboplastin time (aPTT), fibrinogen concentration,
fibrin degradation products, D-dimers, and antithrombin, are required for differentiation
of DIC from thrombotic microangiopathies (TMAs). Additionally, blood pressure should
be considered because HUS usually presents with hypertension. Once DIC has been excluded,
the underlying TMA must be identified. TTP is diagnosed by identification of low ADAMTS13
activity (< 5-10%) and treated urgently with plasma exchange initially; HUS is associated
with normal ADAMTS13 activity (> 5–10%) and the type of HUS elucidated by performing
a Shiga-toxin producing Escherichia coli (STEC) stool culture or polymerase chain
reaction (PCR) assay. Positive STEC strongly suggests STEC-HUS; negative STEC strongly
suggests aHUS, with or without an associated complement-activating condition (e.g.,
infection, malignant hypertension, the post-partum period, kidney transplantation,
drugs, or malignancy). Rapid detection and management of any associated complement-activating
condition and consideration of eculizumab are required [3, 6, 9, 13]
DIC is relatively common, developing in 9–19% of ICU patients, usually as a result
of sepsis [4], with an incidence of 18/100,000 in the overall population [2, 5]. By
contrast, TTP and Shiga-toxin producing Escherichia coli (STEC)-associated HUS have
estimated incidences of 6 and up to 29 cases per million, respectively, and atypical
HUS (aHUS) a prevalence of 0.2–0.4 cases per million [6, 7], making these conditions
far rarer than DIC. Although TTP is described as a pentad of fever, thrombocytopenia,
MAHA, renal dysfunction, and neurological impairment, often some of these features
are not present [7]. Accordingly, TTP may be confused with HUS, which is most commonly
characterized by the triad of thrombocytopenia, MAHA, and renal dysfunction [3]. These
clinical similarities of DIC, TTP, and HUS are a major concern because they pose a
risk of misdiagnosis as intensivists are more likely to consider a diagnosis of DIC
than of the rarer TTP or HUS, thus delaying potentially lifesaving treatment.
Several diagnostic algorithms for TMA have been published [3, 8–10]. However, currently
the only available guidance specific to the ICU are the recently published expert
statements of Azoulay and colleagues [11]. This publication provides an excellent
guide for the differential diagnosis of TMAs but only briefly mentions DIC. A concise
diagnostic algorithm tailored to intensivists would aid rapid differential diagnosis
of TTP and HUS from DIC, and enable early appropriate treatment.
A new algorithm to rapidly differentiate DIC from TTP and HUS in the ICU
Given the importance of differentiating DIC from TTP and HUS, we propose a concise
algorithm based on existing guidance [3, 9, 11] and our own discussions which will
enable the intensivist to rapidly distinguish between these entities (Fig. 1). MAHA,
negative Coombs test, elevated lactate dehydrogenase (LDH) levels, and organ dysfunction
with thrombocytopenia are common to DIC, TTP, and aHUS [2, 3], although patients with
TTP and septic DIC may have more severe thrombocytopenia [2, 12]. The most important
distinguishing factor between DIC and TMAs is the coagulation profile, as patients
with DIC have altered coagulation [2]. However, blood pressure is also important as
HUS often presents with severe hypertension and DIC with hypotension [3, 7]. The combined
evaluation of full blood count and blood smear, hemolysis profile, coagulation profile,
and blood pressure is usually sufficient to ascertain whether a patient has DIC or
a TMA.
Once DIC has been excluded, confirming the cause of the TMA is paramount for appropriate
management. The two most concerning causes of TMA are TTP and HUS. TTP is caused by
a deficiency in a disintegrin-like metalloproteinase with thrombospondin motif type
1 member 13 (ADAMTS13) and has 90% mortality without plasma exchange [7]. HUS is caused
by either Shiga toxin (STEC-HUS) or complement dysregulation as a result of genetic
predisposition or autoantibodies (aHUS) [3, 6, 7, 11]. An ADAMTS13 activity of < 5–10%
is sufficient to confirm TTP [3, 9] and a positive Shiga-toxin stool culture or polymerase
chain reaction (PCR) assay confirms STEC-HUS [3, 9]. In the absence of low ADAMTS13
levels and Shiga-toxin, aHUS, a rare but devastating TMA, is highly likely [6]. Similar
to DIC, aHUS has a rapid onset and non-specific presentation [2, 3]. aHUS can be found
in association with other complement-activating states such as infection, malignant
hypertension, the post-partum period, kidney transplantation, certain drugs, or malignancies
[3]. There can be substantial overlap in the presentation of these conditions and
they may coexist with complement-mediated aHUS, making distinction difficult [3].
It should also be remembered that aHUS can present with malignant hypertension, which
itself can cause TMA [6, 9]. Rapid diagnosis and treatment are essential to prevent
irreversible organ damage and death [13].
Like any pragmatic guidelines, we chose to focus on the most common presentation as
we considered this of most benefit. For comprehensive guidance on TMA diagnosis and
management, we refer to other works, such as those of Scully et al. [7], Campistol
et al. [3], Laurence et al. [9], and Azoulay et al. [11]. While the proposed algorithm
applies to the majority of cases of thrombocytopenia, it must be noted that clinical
judgment and collaboration with experts is essential, as exceptional clinical presentations
do occur [14, 15].
It should also be noted that some of the tests required in the differential diagnosis
(e.g., ADAMTS13 activity assay) are not available at all institutions. If rapid ADAMTS13
testing is not possible, the PLASMIC score, a seven-component prediction tool that
can accurately and reliably predict the probability of severe ADAMTS13 deficiency
[10], can be used. Additionally, we have not included genetic testing for the complement
abnormalities of aHUS in our algorithm; while these can confirm an already suspected
diagnosis of aHUS, the turnaround time is currently considerable and should not be
relied upon in the ICU [11].
Critically ill patients have a range of clinical problems, including multi-organ failure,
sepsis, and shock [5], and early diagnosis and management are crucial to optimize
outcomes. We present a concise diagnostic algorithm that enables intensivists to make
a rapid diagnosis so that they can initiate early appropriate management for ICU patients
with thrombocytopenia. This algorithm adds to the current literature available to
the intensivist [11], with a focus on differentiating TTP and HUS from DIC.
Related collections
Most cited references8
- Record: found
- Abstract: found
- Article: not found
Haemolytic uraemic syndrome
Chantal Loirat, Fadi Fakhouri, Julien Zuber … (2017)

- Record: found
- Abstract: found
- Article: found
Predictive Features of Severe Acquired ADAMTS13 Deficiency in Idiopathic Thrombotic Microangiopathies: The French TMA Reference Center Experience
Paul Coppo, Michaël Schwarzinger, Marc Buffet … (2010)
- Record: found
- Abstract: found
- Article: found
Actualización en síndrome hemolítico urémico atípico: diagnóstico y tratamiento. Documento de consenso
Josep M Campistol, Manuel Arias, Gema Ariceta … (2015)