- Record: found
- Abstract: found
- Article: found
A Non-invasive Platform for Functional Characterization of Stem-Cell-Derived Cardiomyocytes with Applications in Cardiotoxicity Testing

Read this article at
Summary
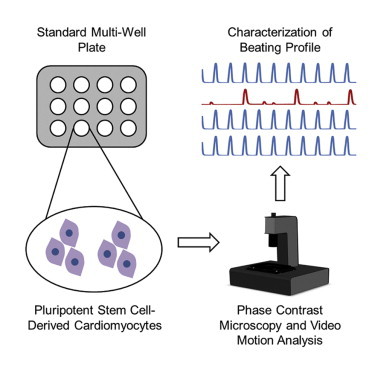
We present a non-invasive method to characterize the function of pluripotent stem-cell-derived cardiomyocytes based on video microscopy and image analysis. The platform, called Pulse, generates automated measurements of beating frequency, beat duration, amplitude, and beat-to-beat variation based on motion analysis of phase-contrast images captured at a fast frame rate. Using Pulse, we demonstrate recapitulation of drug effects in stem-cell-derived cardiomyocytes without the use of exogenous labels and show that our platform can be used for high-throughput cardiotoxicity drug screening and studying physiologically relevant phenotypes.
Graphical Abstract
Highlights
Abstract
In this article, Maddah and colleagues present a non-invasive method to characterize the function of stem-cell-derived cardiomyocytes based on video microscopy and image analysis. They demonstrate automated measurements of beating frequency, duration, amplitude, and variation and show that the platform can be used for cardiotoxicity drug screening and studying physiologically relevant phenotypes.
Related collections
Most cited references18
- Record: found
- Abstract: found
- Article: not found
Cardiomyocytes can be generated from marrow stromal cells in vitro.
- Record: found
- Abstract: found
- Article: not found
Patient-specific induced pluripotent stem cells as a model for familial dilated cardiomyopathy.
- Record: found
- Abstract: found
- Article: not found