- Record: found
- Abstract: found
- Article: not found
Levels of C-peptide, body mass index and age, and their usefulness in classification of diabetes in relation to autoimmunity, in adults with newly diagnosed diabetes in Kronoberg, Sweden
Read this article at
Abstract
Objective
C-peptide is a main outcome measure in treatment trials of diabetes. C-peptide also has a role in the classification of diabetes, which is often difficult in adults and this is also increasingly recognised in adolescents and elders.
Aim
We aimed to describe the levels of C-peptide in relation to age and body mass index (BMI) in a large population-based cohort of adults with newly diagnosed diabetes and compare the capabilities of C-peptide, age and BMI to discriminate between autoimmune and non-autoimmune diabetes.
Subjects and methods
Blood samples from 1180 patients were analysed regarding islet cell antibody, glutamic acid decarboxylase antibody and fasting C-peptide (FCP). Receiver operating characteristics (ROC) curves were analysed to check the ability of age, BMI and C-peptide to discriminate between autoantibody-positive (Ab +) and -negative (Ab −) diabetes.
Results
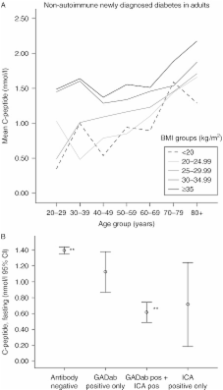
Mean FCP was 0.73±0.5 (range 0.13–1.80) nmol/l in the Ab + and 1.42±0.9 (range 0.13–8.30) nmol/l in the Ab −. FCP was 0.02 nmol/l higher per year increase in age at diagnosis of diabetes. Mean BMI was 26.0±4.8 (range 18.0–39.0) kg/m 2 in the Ab + and 28.9±5.3 (range 15.5–62.6) kg/m 2 in the Ab −. FCP increased with age also within each BMI group. The highest area under the curve (AUC) in the ROC analysis was found for C-peptide, followed by age and BMI (0.78, 0.68 and 0.66 respectively).
Conclusions
At diagnosis of diabetes, C-peptide was superior to age and BMI in discriminating between autoimmune and non-autoimmune diabetes. C-peptide increased significantly with BMI and age, latter also within each BMI group. Most of the adults had normal or high levels of C-peptide at presentation of diabetes among the autoimmune patients.
Related collections
Most cited references40
- Record: found
- Abstract: found
- Article: not found
Age-related changes in total and regional fat distribution.

- Record: found
- Abstract: found
- Article: found
Efficacy and Safety of the Human Glucagon-Like Peptide-1 Analog Liraglutide in Combination With Metformin and Thiazolidinedione in Patients With Type 2 Diabetes (LEAD-4 Met+TZD)
- Record: found
- Abstract: found
- Article: not found
