- Record: found
- Abstract: found
- Article: not found
Effects of mir-21 on Cardiac Microvascular Endothelial Cells After Acute Myocardial Infarction in Rats: Role of Phosphatase and Tensin Homolog (PTEN)/Vascular Endothelial Growth Factor (VEGF) Signal Pathway
Abstract
Background
This study investigated how miR-21 expression is reflected in acute myocardial infarction and explored the role of miR-21 and the PTEN/VEGF signaling pathway in cardiac microvascular endothelial cells.
Material/Methods
We used an in vivo LAD rat model to simulate acute myocardial infarction. MiR-21 mimics and miR-21 inhibitors were injected and transfected into model rats in order to alter miR-21 expression. Cardiac functions were evaluated using echocardiographic measurement, ELISA, and Masson staining. In addition, lenti-PTEN and VEGF siRNA were transfected into CMEC cells using standard procedures for assessing the effect of PTEN and VEGE on cell proliferation, apoptosis, and angiogenesis. MiR-21, PTEN, and VEGF expressions were examined by RT-PCR and Western blot. The relationship between miR-21 and PTEN was determined by the luciferase activity assay.
Results
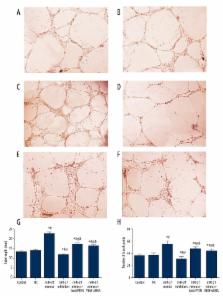
We demonstrated that miR-21 bonded with the 3′-UTR of PTEN and suppressed PTEN expressions. Established models significantly induced cardiac infarct volume and endothelial injury marker expressions as well as miR-21 and PTEN expressions ( P<0.05). MiR-21 mimics exhibited significantly protective effects since they down-regulated both infarction size and injury marker expressions by increasing VEGF expression and inhibiting PTEN expression ( P<0.05). In addition, results from in vitro research show that lenti-PTEN and VEGF siRNA can notably antagonize the effect of miR-21 on cell proliferation, apoptosis, and angiogenesis ( P<0.05).
Related collections
Most cited references36
- Record: found
- Abstract: found
- Article: not found
MicroRNA-92a controls angiogenesis and functional recovery of ischemic tissues in mice.
- Record: found
- Abstract: found
- Article: not found
Cytokines in atherosclerosis: pathogenic and regulatory pathways.
- Record: found
- Abstract: found
- Article: not found
