- Record: found
- Abstract: found
- Article: found
Pathophysiology of reversible cerebral vasoconstriction syndrome
Read this article at
Abstract
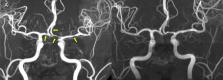
Reversible cerebral vasoconstriction syndrome (RCVS) is a complex neurovascular disorder being recognized during the past two decades. It is characterized by multiple abrupt severe headaches and widespread cerebral vasoconstrictions, with potential complications such as ischemic stroke, convexity subarachnoid hemorrhage, intracerebral hemorrhage and posterior reversible encephalopathy syndrome. The clinical features, imaging findings, and dynamic disease course have been delineated. However, the pathophysiology of RCVS remains elusive. Recent studies have had substantial progress in elucidating its pathogenesis. It is now believed that dysfunction of cerebral vascular tone and impairment of blood–brain barrier may play key roles in the pathophysiology of RCVS, which explains some of the clinical and radiological manifestations of RCVS. Some other potentially important elements include genetic predisposition, sympathetic overactivity, endothelial dysfunction, and oxidative stress, although the detailed molecular mechanisms are yet to be identified. In this review, we will summarize what have been revealed in the literature and elaborate how these factors could contribute to the pathophysiology of RCVS.
Related collections
Most cited references121
- Record: found
- Abstract: found
- Article: not found
Structure and function of the blood-brain barrier.
- Record: found
- Abstract: found
- Article: not found
Circulating angiogenic factors and the risk of preeclampsia.
- Record: found
- Abstract: found
- Article: not found