- Record: found
- Abstract: found
- Article: found
Role of DFNA5 in hearing loss and cancer – a comment on Rakusic et al
letter
15 September 2015
There is no author summary for this article yet. Authors can add summaries to their articles on ScienceOpen to make them more accessible to a non-specialist audience.
Abstract
Dear editor
We would like to comment on the paper published by Rakusic et al about sudden bilateral
hearing loss in gastric cancer as the only symptom of disease.1 The authors state
that “Inactivated DFNA5, otherwise described in hereditary bilateral deafness, perhaps
favors the development of deafness in patients with gastric cancer”.1 We believe this
conclusion is erroneous. Although DFNA5 has been implicated in both hearing loss and
cancer, the underlying molecular mechanisms are different and completely opposite
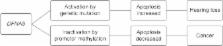
(Figure 1).
In 1998, we identified the first DFNA5 mutation in a Dutch family, as a cause for
a specific form of progressive, sensorineural, and non-syndromic hearing loss.2 This
type of hearing loss is inherited in an autosomal dominant manner. Afterward, other
families were reported with hearing loss due to DFNA5 mutations.3–8 Although all these
DFNA5 mutations are different on DNA level, they all result in skipping of exon 8
on mRNA level, and have an identical effect on the protein.9
The DFNA5 mutation leading to hearing loss is thought to be an activating, gain of
function mutation. As the DFNA5 protein has an apoptosis inducing capacity, the effect
is expected to be an increase in apoptosis, possibly leading to hearing loss by apoptosis
of cells crucial for hearing, such as cochlear hair cells (Figure 1).10
Since 1998, a number of papers on DFNA5 have been published, pointing toward an involvement
in cancer.9–20 Here the molecular mechanism is different. DFNA5 becomes inactivated
through DNA promotor methylation. Because of the inactivation, DFNA5 loses its capacity
to induce apoptosis and most likely contributes to tumorigenesis in this manner (Figure
1).
In conclusion, a very specific gain of function mutation in DFNA5 leads to hearing
loss, while inactivation of DFNA5 on the epigenetic level (DNA methylation) plays
a role in cancer. Therefore, in our opinion, the observed sudden bilateral deafness
in the 60-year-old woman is not caused by inactivation of DFNA5. Akino et al showed
that DFNA5 is methylated in 52% of primary gastric cancers and was correlated with
positivity for Epstein–Barr virus and the absence of metastasis.14 In patients with
metastasized gastric cancer the incidence of DFNA5 methylation was 16.7% (2/12).14
The observation that DFNA5 is inactivated in this woman is thus not exceptional and
in agreement with the literature. However, as described above, inactivation of DFNA5
is very unlikely to be the cause of the observed hearing loss.
Most cited references21
- Record: found
- Abstract: found
- Article: not found
Nonsyndromic hearing impairment is associated with a mutation in DFNA5.
L van Laer, E H Huizing, M Verstreken … (1998)
- Record: found
- Abstract: found
- Article: not found
Aberrant promoter methylation and tumor suppressive activity of the DFNA5 gene in colorectal carcinoma.
M. S. Kim, X. Chang, K Yamashita … (2008)
- Record: found
- Abstract: found
- Article: not found
Identification of DFNA5 as a target of epigenetic inactivation in gastric cancer.
Kimishige Akino, Minoru Toyota, Hiromu Suzuki … (2007)