- Record: found
- Abstract: found
- Article: found
HOIL-1L Interacting Protein (HOIP) as an NF-κB Regulating Component of the CD40 Signaling Complex
Read this article at
Abstract
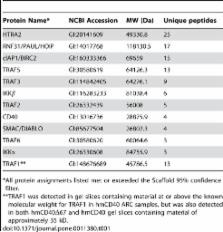
The tumor necrosis factor receptor (TNFR) superfamily mediates signals critical for regulation of the immune system. One family member, CD40, is important for the efficient activation of antibody-producing B cells and other antigen-presenting cells. The molecules and mechanisms that mediate CD40 signaling are only partially characterized. Proteins known to interact with the cytoplasmic domain of CD40 include members of the TNF receptor-associated factor (TRAF) family, which regulate signaling and serve as links to other signaling molecules. To identify additional proteins important for CD40 signaling, we used a combined stimulation/immunoprecipitation procedure to isolate CD40 signaling complexes from B cells and characterized the associated proteins by mass spectrometry. In addition to known CD40-interacting proteins, we detected SMAC/DIABLO, HTRA2/Omi, and HOIP/RNF31/PAUL/ZIBRA. We found that these previously unknown CD40-interacting partners were recruited in a TRAF2-dependent manner. HOIP is a ubiquitin ligase capable of mediating NF-κB activation through the ubiquitin-dependent activation of IKKγ. We found that a mutant HOIP molecule engineered to lack ubiquitin ligase activity inhibited the CD40-mediated activation of NF-κB. Together, our results demonstrate a powerful approach for the identification of signaling molecules associated with cell surface receptors and indicate an important role for the ubiquitin ligase activity of HOIP in proximal CD40 signaling.
Related collections
Most cited references22
- Record: found
- Abstract: found
- Article: not found
Identification of DIABLO, a mammalian protein that promotes apoptosis by binding to and antagonizing IAP proteins.
- Record: found
- Abstract: found
- Article: not found
Nonredundant and complementary functions of TRAF2 and TRAF3 in a ubiquitination cascade that activates NIK-dependent alternative NF-kappaB signaling.
- Record: found
- Abstract: found
- Article: not found