- Record: found
- Abstract: found
- Article: found
Enhanced brightness of bacterial luciferase by bioluminescence resonance energy transfer
Read this article at
Abstract
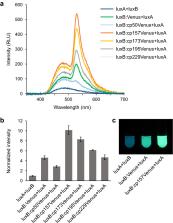
Using the lux operon ( luxCDABE) of bacterial bioluminescence system as an autonomous luminous reporter has been demonstrated in bacteria, plant and mammalian cells. However, applications of bacterial bioluminescence-based imaging have been limited because of its low brightness. Here, we engineered the bacterial luciferase (heterodimer of luxA and luxB) by fusion with Venus, a bright variant of yellow fluorescent protein, to induce bioluminescence resonance energy transfer (BRET). By using decanal as an externally added substrate, color change and ten-times enhancement of brightness was achieved in Escherichia coli when circularly permuted Venus was fused to the C-terminus of luxB. Expression of the Venus-fused luciferase in human embryonic kidney cell lines (HEK293T) or in Nicotiana benthamiana leaves together with the substrate biosynthesis-related genes ( luxC, luxD and luxE) enhanced the autonomous bioluminescence. We believe the improved luciferase will forge the way towards the potential development of autobioluminescent reporter system allowing spatiotemporal imaging in live cells.
Related collections
Most cited references31
- Record: found
- Abstract: found
- Article: not found
Expanded dynamic range of fluorescent indicators for Ca(2+) by circularly permuted yellow fluorescent proteins.
- Record: found
- Abstract: found
- Article: not found
A variant of yellow fluorescent protein with fast and efficient maturation for cell-biological applications.
- Record: found
- Abstract: found
- Article: not found