- Record: found
- Abstract: found
- Article: found
Expression of oestrogen receptors, ERα, ERβ, and ERβ variants, in endometrial cancers and evidence that prostaglandin F may play a role in regulating expression of ERα
Read this article at
Abstract
Background
Endometrial cancer is the most common gynaecological malignancy; risk factors include exposure to oestrogens and high body mass index. Expression of enzymes involved in biosynthesis of oestrogens and prostaglandins (PG) is often higher in endometrial cancers when compared with levels detected in normal endometrium. Oestrogens bind one of two receptors (ERα and ERβ) encoded by separate genes. The full-length receptors function as ligand-activated transcription factors; splice variant isoforms of ERβ lacking a ligand-binding domain have also been described. PGs act in an autocrine or paracrine manner by binding to specific G-protein coupled receptors.
Methods
We compared expression of ERs, progesterone receptor (PR) and cyclooxygenase-2 (COX-2) in stage 1 endometrial adenocarcinomas graded as well (G1), moderately (G2) or poorly (G3) differentiated (n ≥ 10 each group) using qRTPCR, single and double immunohistochemistry. We used endometrial adenocarcinoma cell lines to investigate the impact of PGF2α on expression of ERs and PR.
Results
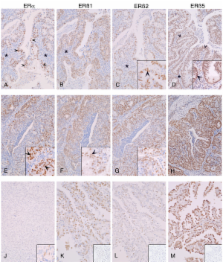
Full length ERβ (ERβ1) and two ERβ variants (ERβ2, ERβ5) were expressed in endometrial cancers regardless of grade and the proteins were immunolocalised to the nuclei of cells in both epithelial and stromal compartments. Immunoexpression of COX-2 was most intense in cells that were ERα neg/low. Expression of PR in endometrial adenocarcinoma (Ishikawa) cell lines and tissues broadly paralleled that of ERα. Treatment of adenocarcinoma cells with PGF2α reduced expression of ERα but had no impact on ERβ1. Cells incubated with PGF2α were unable to increase expression of PR mRNA when they were incubated with E2.
Conclusion
We have demonstrated that ERβ5 protein is expressed in stage 1 endometrial adenocarcinomas. Expression of three ERβ variants, including the full-length protein is not grade-dependent and most cells in poorly differentiated cancers are ERβ pos/ERα neg. We found evidence of a link between COX-2, its product PGF2α, and expression of ERα and PR that sheds new light on the cross talk between steroid and PG signalling pathways in this disease.
Related collections
Most cited references62
- Record: found
- Abstract: found
- Article: not found
Differential ligand activation of estrogen receptors ERalpha and ERbeta at AP1 sites.
- Record: found
- Abstract: found
- Article: not found