- Record: found
- Abstract: found
- Article: found
Liver Fibrosis in Non-alcoholic Fatty Liver Disease: From Liver Biopsy to Non-invasive Biomarkers in Diagnosis and Treatment
Read this article at
Abstract
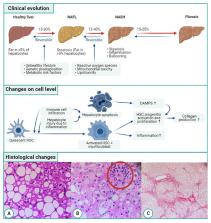
An increasing percentage of people have or are at risk to develop non-alcoholic fatty liver disease (NAFLD) worldwide. NAFLD comprises different stadia going from isolated steatosis to non-alcoholic steatohepatitis (NASH). NASH is a chronic state of liver inflammation that leads to the transformation of hepatic stellate cells to myofibroblasts. These cells produce extra-cellular matrix that results in liver fibrosis. In a normal situation, fibrogenesis is a wound healing process that preserves tissue integrity. However, sustained and progressive fibrosis can become pathogenic. This process takes many years and is often asymptomatic. Therefore, patients usually present themselves with end-stage liver disease e.g., liver cirrhosis, decompensated liver disease or even hepatocellular carcinoma. Fibrosis has also been identified as the most important predictor of prognosis in patients with NAFLD. Currently, only a minority of patients with liver fibrosis are identified to be at risk and hence referred for treatment. This is not only because the disease is largely asymptomatic, but also due to the fact that currently liver biopsy is still the golden standard for accurate detection of liver fibrosis. However, performing a liver biopsy harbors some risks and requires resources and expertise, hence is not applicable in every clinical setting and is unsuitable for screening. Consequently, different non-invasive diagnostic tools, mainly based on analysis of blood or other specimens or based on imaging have been developed or are in development. In this review, we will first give an overview of the pathogenic mechanisms of the evolution from isolated steatosis to fibrosis. This serves as the basis for the subsequent discussion of the current and future diagnostic biomarkers and anti-fibrotic drugs.
Related collections
Most cited references245
- Record: found
- Abstract: found
- Article: not found
The NLRP3 inflammasome: molecular activation and regulation to therapeutics
- Record: found
- Abstract: found
- Article: not found