- Record: found
- Abstract: found
- Article: found
Skeletal intramyocellular lipid metabolism and insulin resistance
Read this article at
Abstract
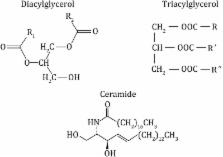
Lipids stored in skeletal muscle cells are known as intramyocellular lipid (IMCL). Disorders involving IMCL and its causative factor, circulatory free fatty acids (FFAs), induce a toxic state and ultimately result in insulin resistance (IR) in muscle tissue. On the other hand, intramuscular triglyceride (IMTG), the most abundant component of IMCL and an essential energy source for active skeletal muscle, is different from other IMCLs, as it is stored in lipid droplets and plays a pivotal role in skeletal muscle energy homeostasis. This review discusses the association of FFA-induced ectopic lipid accumulation and IR, with specific emphasis on the relationship between IMCL/IMTG metabolism and IR.
Related collections
Most cited references59
- Record: found
- Abstract: found
- Article: not found
FAT SIGNALS - Lipases and Lipolysis in Lipid Metabolism and Signaling

- Record: found
- Abstract: found
- Article: found
Skeletal Muscle Triglycerides, Diacylglycerols, and Ceramides in Insulin Resistance
- Record: found
- Abstract: found
- Article: not found