- Record: found
- Abstract: found
- Article: found
Insights into plant cell wall structure, architecture, and integrity using glycome profiling of native and AFEX TM-pre-treated biomass
Read this article at
Highlight
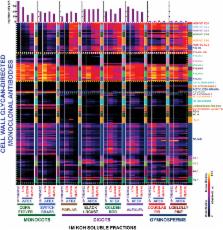
Glycome profiling and AFEX pre-treatment reveal differences in cell wall architecture among different plant taxa and implicate subclasses of polysaccharides whose loosening results in reduced recalcitrance of walls to deconstruction.
Abstract
Cell walls, which constitute the bulk of plant biomass, vary considerably in their structure, composition, and architecture. Studies on plant cell walls can be conducted on both native and pre-treated plant biomass samples, allowing an enhanced understanding of these structural and compositional variations. Here glycome profiling was employed to determine the relative abundance of matrix polysaccharides in several phylogenetically distinct native and pre-treated plant biomasses. Eight distinct biomass types belonging to four different subgroups (i.e. monocot grasses, woody dicots, herbaceous dicots, and softwoods) were subjected to various regimes of AFEX™ (ammonia fiber expansion) pre-treatment [AFEX is a trademark of MBI, Lansing ( http://www.mbi.org]. This approach allowed detailed analysis of close to 200 cell wall glycan epitopes and their relative extractability using a high-throughput platform. In general, irrespective of the phylogenetic origin, AFEX™ pre-treatment appeared to cause loosening and improved accessibility of various xylan epitope subclasses in most plant biomass materials studied. For most biomass types analysed, such loosening was also evident for other major non-cellulosic components including subclasses of pectin and xyloglucan epitopes. The studies also demonstrate that AFEX™ pre-treatment significantly reduced cell wall recalcitrance among diverse phylogenies (except softwoods) by inducing structural modifications to polysaccharides that were not detectable by conventional gross composition analyses. It was found that monitoring changes in cell wall glycan compositions and their relative extractability for untreated and pre-treated plant biomass can provide an improved understanding of variations in structure and composition of plant cell walls and delineate the role(s) of matrix polysaccharides in cell wall recalcitrance.
Related collections
Most cited references28
- Record: found
- Abstract: found
- Article: not found
Cell-wall carbohydrates and their modification as a resource for biofuels.
- Record: found
- Abstract: found
- Article: not found
How does plant cell wall nanoscale architecture correlate with enzymatic digestibility?
- Record: found
- Abstract: found
- Article: not found