- Record: found
- Abstract: found
- Article: found
The emerging role of sorting nexins in cardiovascular diseases
review-article
Read this article at
There is no author summary for this article yet. Authors can add summaries to their articles on ScienceOpen to make them more accessible to a non-specialist audience.
Abstract
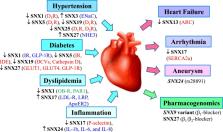
The sorting nexin (SNX) family consists of a diverse group of cytoplasmic- and membrane-associated phosphoinositide-binding proteins that play pivotal roles in the regulation of protein trafficking. This includes the entire endocytic pathway, such as endocytosis, endosomal sorting, and endosomal signaling. Dysfunctions of SNX pathway are involved in several forms of cardiovascular disease (CVD). Moreover, SNX gene variants are associated with CVDs. In this review, we discuss the current knowledge on SNX-mediated regulatory mechanisms and their roles in the pathogenesis and treatment of CVDs.
Related collections
Most cited references90
- Record: found
- Abstract: found
- Article: not found
Endosomal sorting and signalling: an emerging role for sorting nexins.
Peter J. Cullen (2008)
- Record: found
- Abstract: found
- Article: not found
Insights into the PX (phox-homology) domain and SNX (sorting nexin) protein families: structures, functions and roles in disease.
Leonard Collins, M. D. Teasdale (2012)
- Record: found
- Abstract: found
- Article: not found
Modulation of receptor recycling and degradation by the endosomal kinesin KIF16B.
Harald Stenmark, Anja Runge, Alicia Cabezas … (2005)