- Record: found
- Abstract: found
- Article: found
Impaired Transcriptional Activity of Nrf2 in Age-Related Myocardial Oxidative Stress Is Reversible by Moderate Exercise Training
Read this article at
Abstract
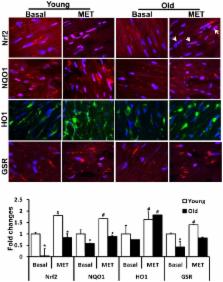
Aging promotes accumulation of reactive oxygen/nitrogen species (ROS/RNS) in cardiomyocytes, which leads to contractile dysfunction and cardiac abnormalities. These changes may contribute to increased cardiovascular disease in the elderly. Inducible antioxidant pathways are regulated by nuclear erythroid 2 p45-related factor 2 (Nrf2) through antioxidant response cis-elements (AREs) and are impaired in the aging heart. Whereas acute exercise stress (AES) activates Nrf2 signaling and promotes myocardial antioxidant function in young mice (∼2 months), aging mouse (>23 months) hearts exhibit significant oxidative stress as compared to those of the young. The purpose of this study was to investigate age-dependent regulation of Nrf2-antioxidant mechanisms and redox homeostasis in mouse hearts and the impact of exercise. Old mice were highly susceptible to oxidative stress following high endurance exercise stress (EES), but demonstrated increased adaptive redox homeostasis after moderate exercise training (MET; 10m/min, for 45 min/day) for ∼6 weeks. Following EES, transcription and protein levels for most of the ARE-antioxidants were increased in young mice but their induction was blunted in aging mice. In contrast, 6-weeks of chronic MET promoted nuclear levels of Nrf2 along with its target antioxidants in the aging heart to near normal levels as seen in young mice. These observations suggest that enhancing Nrf2 function and endogenous cytoprotective mechanisms by MET, may combat age-induced ROS/RNS and protect the myocardium from oxidative stress diseases.
Related collections
Most cited references47
- Record: found
- Abstract: found
- Article: not found
Brusatol enhances the efficacy of chemotherapy by inhibiting the Nrf2-mediated defense mechanism.
- Record: found
- Abstract: found
- Article: not found
Decline in transcriptional activity of Nrf2 causes age-related loss of glutathione synthesis, which is reversible with lipoic acid.
- Record: found
- Abstract: found
- Article: not found