- Record: found
- Abstract: found
- Article: found
Macrophage deficiency of miR‐21 promotes apoptosis, plaque necrosis, and vascular inflammation during atherogenesis
Read this article at
Abstract
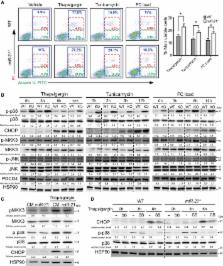
Atherosclerosis, the major cause of cardiovascular disease, is a chronic inflammatory disease characterized by the accumulation of lipids and inflammatory cells in the artery wall. Aberrant expression of micro RNAs has been implicated in the pathophysiological processes underlying the progression of atherosclerosis. Here, we define the contribution of miR‐21 in hematopoietic cells during atherogenesis. Interestingly, we found that miR‐21 is the most abundant mi RNA in macrophages and its absence results in accelerated atherosclerosis, plaque necrosis, and vascular inflammation. miR‐21 expression influences foam cell formation, sensitivity to ER‐stress‐induced apoptosis, and phagocytic clearance capacity. Mechanistically, we discovered that the absence of miR‐21 in macrophages increases the expression of the miR‐21 target gene, MKK3, promoting the induction of p38‐ CHOP and JNK signaling. Both pathways enhance macrophage apoptosis and promote the post‐translational degradation of ABCG1, a transporter that regulates cholesterol efflux in macrophages. Altogether, these findings reveal a major role for hematopoietic miR‐21 in atherogenesis.
Related collections
Most cited references41
- Record: found
- Abstract: found
- Article: not found
MiR-33 contributes to the regulation of cholesterol homeostasis.
- Record: found
- Abstract: found
- Article: not found