- Record: found
- Abstract: found
- Article: found
Characterization of mouse orofacial pain and the effects of lesioning TRPV1-expressing neurons on operant behavior
Read this article at
Abstract
Background
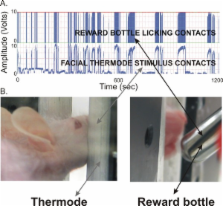
Rodent models of orofacial pain typically use methods adapted from manipulations to hind paw; however, limitations of these models include animal restraint and subjective assessments of behavior by the experimenter. In contrast to these methods, assessment of operant responses to painful stimuli has been shown to overcome these limitations and expand the breadth of interpretation of the behavioral responses. In the current study, we used an operant model based on a reward-conflict paradigm to assess nociceptive responses in three strains of mice (SKH1-Hr hr, C57BL/6J, TRPV1 knockout). We previously validated this operant model in rats and hypothesized in this study that wild-type mice would demonstrate a similar thermal stimulus-dependent response and similar operant pain behaviors. Additionally, we evaluated the effects on operant behaviors of mice manipulated genetically (e.g., TRPV1 k.o.) or pharmacologically with resiniferatoxin (RTX), a lesioning agent for TRPV1-expressing neurons. During the reward-conflict task, mice accessed a sweetened milk reward solution by voluntarily position their face against a neutral or heated thermode (37–55°C).
Results
As the temperature of the thermal stimulus became noxiously hot, reward licking events in SKH1-Hr hr and C57BL/6J mice declined while licking events in TRPV1 k.o. mice were insensitive to noxious heat within the activation range of TRPV1 (37–52°C). All three strains displayed nocifensive behaviors at 55°C, as indicated by a significant decrease in reward licking events. Induction of neurogenic inflammation by topical application of capsaicin reduced licking events in SKH1-Hr hr mice, and morphine rescued this response. Again, these results parallel what we previously documented using rats in this operant system. Following intracisternal treatment with RTX, C57BL/6J mice demonstrated a block of noxious heat at both 48 and 55°C. RTX-treated TRPV1 k.o. mice and all vehicle-treated mice displayed similar reward licking events as compared to the pre-treatment baseline levels. Both TRPV1 k.o. and RTX-treated C57BL/6J had complete abolishment of eye-wipe responses following corneal application of capsaicin.
Related collections
Most cited references50
- Record: found
- Abstract: found
- Article: not found
Impaired nociception and pain sensation in mice lacking the capsaicin receptor.
- Record: found
- Abstract: found
- Article: not found
TRPM8 is required for cold sensation in mice.
- Record: found
- Abstract: found
- Article: not found