- Record: found
- Abstract: found
- Article: not found
Long, thin transmission chains of Severe Acute Respiratory Syndrome Coronavirus 2 may go undetected for several weeks at low to moderate reproduction numbers: Implications for containment and elimination strategy
Read this article at
Abstract
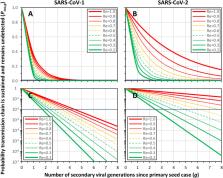
Severe Acute Respiratory Syndrome Coronavirus 1 (SARS-CoV-1) infections almost always caused overt symptoms, so effective case and contact management enabled its effective eradication within months. However, Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) usually causes only mild symptoms, so transmission chains may grow to include several individuals before at least one index case becomes ill enough to self-report for diagnosis and care. Here, simple mathematical models were developed to evaluate the implications of delayed index case detection for retrospective contact tracing and management responses. Specifically, these simulations illustrate how: (1) Contact tracing and management may effectively contain most but not all large SARS-CoV-2 clusters arising at foci with high reproduction numbers because rapidly expanding transmission chains ensure at least one overtly symptomatic index case occurs within two viral generations a week or less apart. (2) However, lower reproduction numbers give rise to thinner transmission chains extending through longer sequences of non-reporting asymptomatic and paucisymptomatic individuals, often spanning three or more viral generations (≥2 weeks of transmission) before an overtly symptomatic index case occurs. (3) Consequently, it is not always possible to fully trace and contain such long, thin transmission chains, so the community transmission they give rise to is underrepresented in surveillance data. (4) Wherever surveillance systems are weak and/or transmission proceeds within population groups with lower rates of overt clinical symptoms and/or self-reporting, case and contact management effectiveness may be more severely limited, even at the higher reproduction numbers associated with larger outbreaks. (5) Because passive surveillance platforms may be especially slow to detect the thinner transmission chains that occur at low reproduction numbers, establishing satisfactory confidence of elimination may require that no confirmed cases are detected for two full months, throughout which presumptive preventative measures must be maintained to ensure complete collapse of undetected residual transmission. (6) Greater scope exists for overcoming these limitations by enhancing field surveillance for new suspected cases than by improving diagnostic test sensitivity. (7) While population-wide active surveillance may enable complete traceability and containment, this goal may also be achievable through enhanced passive surveillance for paucisymptomatic infections, combining readily accessible decentralized testing with population hypersensitization to self-reporting with mild symptoms. Containment and elimination of SARS-CoV-2 will rely far more upon presumptive, population-wide prevention measures than was necessary for SARS-CoV-1, necessitating greater ambition, political will, investment, public support, persistence and patience. Nevertheless, case and contact management may be invaluable for at least partially containing SARS-CoV-2 transmission, especially larger outbreaks, but only if enabled by sufficiently sensitive surveillance. Furthermore, consistently complete transmission chain containment may be enabled by focally enhanced surveillance around manageably small numbers of outbreaks in the end stages of successful elimination campaigns, so that their endpoints may be accelerated and sustained.
Related collections
Most cited references45
- Record: found
- Abstract: found
- Article: found
Physical distancing, face masks, and eye protection to prevent person-to-person transmission of SARS-CoV-2 and COVID-19: a systematic review and meta-analysis

- Record: found
- Abstract: found
- Article: found
Substantial undocumented infection facilitates the rapid dissemination of novel coronavirus (SARS-CoV2)

- Record: found
- Abstract: found
- Article: found
