- Record: found
- Abstract: found
- Article: found
Characterization of the expression of gastrin-releasing peptide and its receptor in the trigeminal and spinal somatosensory systems of Japanese macaque monkeys: Insight into humans
Read this article at
Abstract
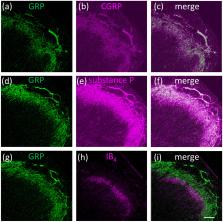
Gastrin-releasing peptide (GRP) and its receptor (GRPR) have been identified as itch mediators in the spinal and trigeminal somatosensory systems in rodents. In primates, there are few reports of GRP/GRPR expression or function in the spinal sensory system and virtually nothing is known in the trigeminal system. The aim of the present study was to characterize GRP and GRPR in the trigeminal and spinal somatosensory system of Japanese macaque monkeys ( Macaca fuscata). cDNA encoding GRP was isolated from the macaque dorsal root ganglion (DRG) and exhibited an amino acid sequence that was highly conserved among mammals and especially in primates. Immunohistochemical analysis demonstrated that GRP was expressed mainly in the small-sized trigeminal ganglion and DRG in adult macaque monkeys. Densely stained GRP-immunoreactive (ir) fibers were observed in superficial layers of the spinal trigeminal nucleus caudalis (Sp5C) and the spinal cord. In contrast, GRP-ir fibers were rarely observed in the principal sensory trigeminal nucleus and oral and interpolar divisions of the spinal trigeminal nucleus. cDNA cloning, in situ hybridization, and Western blot revealed substantial expression of GRPR mRNA and GRPR protein in the macaque spinal dorsal horn and Sp5C. Our Western ligand blot and ligand derivative stain for GRPR revealed that GRP directly bound in the macaque Sp5C and spinal dorsal horn as reported in rodents. Finally, GRP-ir fibers were also detected in the human spinal dorsal horn. The spinal and trigeminal itch neural circuits labeled with GRP and GRPR appear to function also in primates.
Related collections
Most cited references49
- Record: found
- Abstract: found
- Article: not found
Vascularized and functional human liver from an iPSC-derived organ bud transplant.
- Record: found
- Abstract: found
- Article: not found
Differential distribution of calcitonin gene-related peptide and its receptor components in the human trigeminal ganglion.
- Record: found
- Abstract: found
- Article: not found
