- Record: found
- Abstract: found
- Article: found
Influence of physical properties of carrier on the performance of dry powder inhalers
Read this article at
Abstract
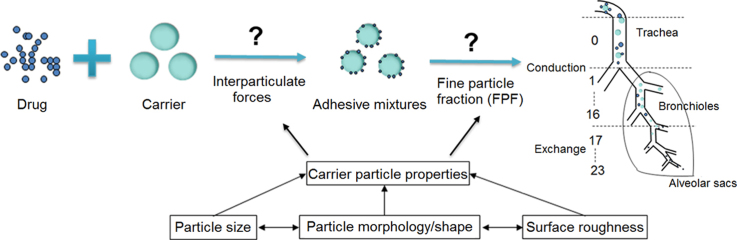
Dry powder inhalers (DPIs) offer distinct advantages as a means of pulmonary drug delivery and have attracted much attention in the field of pharmaceutical science. DPIs commonly contain micronized drug particles which, because of their cohesiveness and strong propensity to aggregate, have poor aerosolization performance. Thus carriers with a larger particle size are added to address this problem. However, the performance of DPIs is profoundly influenced by the physical properties of the carrier, particularly their particle size, morphology/shape and surface roughness. Because these factors are interdependent, it is difficult to completely understand how they individually influence DPI performance. The purpose of this review is to summarize and illuminate how these factors affect drug–carrier interaction and influence the performance of DPIs.
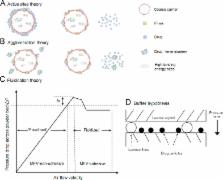
Graphical abstract
The physical properties of carrier particles used in dry powder inhalers (DPIs) particularly their size, morphology/shape, and surface roughness play a significant role in determining DPI performance by influencing the adhesion and detachment of drug–carrier adhesive mixtures. However, it is difficult to completely understand how they influence DPI performance as they are interdependent.
Related collections
Most cited references81
- Record: found
- Abstract: found
- Article: not found
Inhaling medicines: delivering drugs to the body through the lungs.
- Record: found
- Abstract: not found
- Article: not found
The relationship between attractive interparticle forces and bulk behaviour in dry and uncharged fine powders
- Record: found
- Abstract: found
- Article: not found