- Record: found
- Abstract: found
- Article: found
Increasing fetal ovine number per gestation alters fetal plasma clinical chemistry values
Read this article at
Abstract
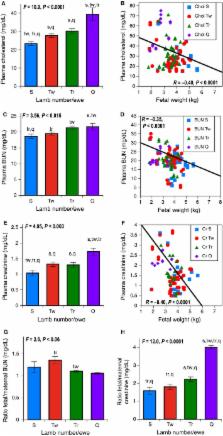
Intrauterine growth restriction ( IUGR) is interconnected with developmental programming of lifelong pathophysiology. IUGR is seen in human multifetal pregnancies, with stepwise rises in fetal numbers interfering with placental nutrient delivery. It remains unknown whether fetal blood analyses would reflect fetal nutrition, liver, and excretory function in the last trimester of human or ovine IUGR. In an ovine model, we hypothesized that fetal plasma biochemical values would reflect progressive placental, fetal liver, and fetal kidney dysfunction as the number of fetuses per gestation rose. To determine fetal plasma biochemical values in singleton, twin, triplet, and quadruplet/quintuplet ovine gestation, we investigated morphometric measures and comprehensive metabolic panels with nutritional measures, liver enzymes, and placental and fetal kidney excretory measures at gestational day ( GD) 130 (90% gestation). As anticipated, placental dysfunction was supported by a stepwise fall in fetal weight, fetal plasma glucose, and triglyceride levels as fetal number per ewe rose. Fetal glucose and triglycerides were directly related to fetal weight. Plasma creatinine, reflecting fetal renal excretory function, and plasma cholesterol, reflecting placental excretory function, were inversely correlated with fetal weight. Progressive biochemical disturbances and growth restriction accompanied the rise in fetal number. Understanding the compensatory and adaptive responses of growth‐restricted fetuses at the biochemical level may help explain how metabolic pathways in growth restriction can be predetermined at birth. This physiological understanding is important for clinical care and generating interventional strategies to prevent altered developmental programming in multifetal gestation.
Related collections
Most cited references28
- Record: found
- Abstract: found
- Article: not found
The developmental origins of adult disease (Barker) hypothesis.
- Record: found
- Abstract: found
- Article: not found