- Record: found
- Abstract: found
- Article: found
microRNA‐148a‐3p in extracellular vesicles derived from bone marrow mesenchymal stem cells suppresses SMURF1 to prevent osteonecrosis of femoral head

Read this article at
Abstract
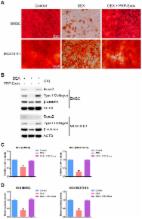
Extracellular vesicle (EV)‐associated microRNAs (miRNAs) have been found as the important biomarkers participating in the development of osteonecrosis of the femoral head (ONFH). Consequently, this study sought to examine the underlying mechanism of bone marrow mesenchymal stem cell (BMSC)‐derived EVs containing miR‐148a‐3p in ONFH. The ONFH rat models were established. Reverse transcription quantitative polymerase chain reaction (RT‐qPCR) and Western blot analysis were applied to detect miR‐148a‐3p, Smad ubiquitination regulatory factor 1 (SMURF1), SMAD7 and B‐cell CLL/lymphoma 2 (BCL2) expression, followed by determination of relationship between miR‐148a‐3p and SMURF1. BMSCs were isolated from normal rats and ONFH rats, and EVs were extracted from BMSCs of normal rats. BMSCs from ONFH rats were treated with mimic, inhibitor, small interfering RNA or EVs from miR‐148a‐3p mimic‐treated BMSCs from normal rats (BMSC‐EV‐miR‐148a‐3p mimic). Cell Counting Kit‐8 and alizarin red staining were utilized to detect cell viability and osteogenic differentiation of BMSCs. ONFH rats were injected with BMSC‐EV‐miR‐148a‐3p mimic to explore the function of BMSC‐EV‐delivered miR‐148a‐3p in vivo. miR‐148a‐3p was down‐regulated in BMSCs and EVs from ONFH rats following decreased BMSCs viability and osteogenic differentiation. SMURF1 was a target gene of miR‐148a‐3p, and resulted in ubiquitination and degradation of SMAD7 to decreased BCL2 expression. The proliferation and differentiation of BMSCs were promoted by BMSC‐EV‐miR‐148a‐3p mimic or SMURF1 silencing. Additionally, BMSC‐EV‐miR‐148a‐3p mimic increased cell proliferation and osteogenic response, diminished SMURF1 expression, and elevated SMAD7 and BCL2 expression in ONFH rats. Collectively, miR‐148a‐3p overexpressed in BMSC‐EVs promoted SMAD7 and BCL2 expression by inhibiting SMURF1, thus alleviating ONFH.
Related collections
Most cited references43

- Record: found
- Abstract: found
- Article: found
Mesenchymal Stem Cell‐Derived Extracellular Vesicles as Mediators of Anti‐Inflammatory Effects: Endorsement of Macrophage Polarization

- Record: found
- Abstract: found
- Article: found