- Record: found
- Abstract: found
- Article: not found
Dynamically Chiral Helical Polymers: A New Frontier in Asymmetric Catalysis?
news
Read this article at
There is no author summary for this article yet. Authors can add summaries to their articles on ScienceOpen to make them more accessible to a non-specialist audience.
Abstract
The impact
of asymmetric catalysis
for the synthesis of enantiomerically enriched compounds cannot be
overstated. In view of the importance of chiral compounds in myriad
applications including agrochemicals, pharmaceuticals, flavors and
fragrances, sensors, and functional materials, the synthesis of these
compounds in single enantiomer form represents a continuing challenge.
In the never-ending search for new methods and concepts to effect
asymmetric synthesis, Suginome et al. have introduced a novel approach
that leverages the cumulative effect of small perturbations provided
by chiral solvents to induce a helical conformation in a polymer that
subsequently acts as an enantioselective catalyst for a number of
useful transformations.
1
In 1848, a
26-year old Louis Pasteur made the first attempt to
find a causal connection between a macroscopic phenomenon (optical
rotation) and molecular structure (dissymmetry). Although a full rationalization
would have to wait until 1874 when the theory of tetrahedral carbon
was formulated, this connection between the molecular and macroscopic
worlds forms the foundation for the sui generis character
of chemistry.
Just as Pasteur recognized that macroscopic chirality,
i.e., optical
rotation or hemihedral crystal faces, can arise from both dissymmetric (e.g.,
tartrates) and symmetric (e.g. silica) molecular building blocks, so too
can mesoscopic objects be constructed from both kinds of molecular
entities. Nowhere is this phenomenon more dramatically manifest than
in the field of materials-chirality.
2
The
vast majority of polymers adopt chiral architectures ranging from
isotactic polypropylene to DNA. However, whereas the chirality of
these polymers arises from the stereogenic centers in the main chain
or the chirality of the subunits (nucleotides), other polymers can
adopt chiral architectures from the induced conformation of the backbone
constructed from achiral subunits. The most common chiral architecture
is helicity, and the sense of helicity can be controlled in a number
of ways. Most commonly, attachment of stereogenic, chirotopic groups
to the monomers is very effective at controlling the helicity of a
polymer. However, as first shown by Green in 1993, chiral media, such
as solvents, can also induce a specific sense of helicity.
3
The use of chiral solvents to control the
stereochemical course
of reactions has been studied for decades.
4
However, these solvents are designed to effect specific interactions
with substrate molecules making them rather unique, engineered, and
expensive. Moreover, for inexpensive, readily available chiral solvents
to be of general utility, they cannot contain many reactive functional
groups. Accordingly, the interactions that such compounds can engender
are likely to be rather weak and nonspecific. This problem is elegantly
addressed by the cumulative effect of small energetic contributions
that leads to the folding of polymers.
5
Most importantly, if the polymer helicity is dynamic, then the enantiomeric
composition of the polymer will be dependent on the degree of polymerization
(DP) which allows the small contributions to accumulate to a significant
energetic bias for single handedness.
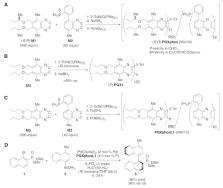
In previous studies, Suginome
and co-workers have used stereogenic
side chains to bias the helicity of poly(quinoxaline-2,3-diyl) polymers
(Scheme 1
).
6
Through copolymerization of the chiral monomer
(R,R)-M1 with a phosphine-bearing
monomer M2, a helically chiral polymer (R,R)-PQXphos (950/50) is generated that
is highly effective in various enantioselective reactions using palladium
catalysts (including the Suzuki–Miyaura cross-coupling). Furthermore,
the authors made the striking observation that the screw sense of
the polymer was solvent dependent. This observation, together with
the known influence of chiral solvents on polymer helicity,
7
inspired the work described in this publication.
1
Scheme 1
To create a chiral polymer with high helical
homogeneity [specified
as screw excess (% se)] using achiral monomers, the authors first
evaluated the polymerization of 1,2-diisocyanobenzene monomers bearing
five different side chains in five different, commercially available
chiral solvents. These experiments led to the identification of the n-propoxymethyl
side chain (M3) and (R)-limonene as the superior combination reaching a maximum
of 72% se for the right-handed helix (P)-PQX1. To create a helically homogeneous polymer,
the authors evaluated
the screw excess of (P)-PQX1 as a function
of the degree of polymerization which reached >99% se at DP ≥
120 (Scheme 1
B). Next,
following their previous protocols, random copolymers of M3 and phosphine M2 were
prepared with DP = 1000, and
this polymer, PQXphosL1 (990/10), was employed as a ligand
for various palladium-catalyzed reactions (Scheme 1
C).
To evaluate the efficiency
of PQXphosL1 (990/10) in
the Suzuki–Miyaura cross-coupling reaction, the ligand was
combined with a palladium catalyst precursor and tested in the reaction
between bromide 1 and boronic acid 2 (Scheme 1
D). By carrying out
the cross-coupling in a 95:5 mixture of (R)-limonene
and THF, the 1,1-binaphthyl product 3 was generated in
66% yield and with 98% ee of (S) absolute configuration.
A number of careful control experiments in the same solvent mixture
using polystyrene-based phosphines as well as monomeric phosphines
failed to produce 3 or produced it as a racemic mixture
thus confirming the critical role of the helical polymer in controlling
the stereochemical course of the reaction.
With the exponential
increase in the development of ligands and
other chiral compounds for application in asymmetric catalysis, a
parallel effort in the practical application of these methods has
also increased. In particular, the adoption of asymmetric catalytic
reactions in industrial processes has lagged behind owing to many
factors, including cost which could be ameliorated by recoverability
and reusability of precious catalysts. This motivation has provided
the impetus for extensive research into immobilization of small molecule
catalysts on surfaces, in polymers (both soluble and insoluble) and
on inorganic supports.
8
It is in this context
that the contribution from Suginome et al. should be evaluated. Although
it would appear at first glance to be a nonstarter for fine chemical
synthesis, limonene is used industrially as a degreasing agent and
paint stripper. The demand for limonene is growing in view of the
trend toward renewable, biobased solvents. Nevertheless, cost will
still be a major deterrent for the near term. In recognition of this
potential problem, Suginome and co-workers did isolate PQXphosL1 (990/10) by precipitation
from (R)-limonene with
methanol and used this material in the Suzuki–Miyaura cross-coupling
in an achiral solvent (THF). The product, 3, was formed
in comparable yield but only 45% ee whereas, in 1-propanol (in which
the ligand is much less soluble), 3 was formed in 88%
ee. Thus, the dynamic character of the helical chirality which allows
for the formation of highly screw enriched polymers is also a major
liability when used in normal solvents.
When compared to the performance
of the helically chiral ligands
such as (R,R)-PQXphos (950/50) in reactions carried out in achiral solvents, the ligand
derived from achiral monomers performs at approximately the same level
in both yield and enantioselectivity. Thus, from a purely practical
perspective, the use of the dynamically chiral ligands does not offer
obvious advantages. However, from a conceptual perspective, this work
constitutes an exceptionally novel application of dynamic polymer
structure and also provides fundamental insights into the fascinating
world of materials-chirality.
One final word about nomenclature
is in order. The authors have,
for the most part, eschewed the commonly used and technically incorrect
neologism of “chirality transfer” and “memory
of chirality”. As has been eloquently pointed out by Cozzi
and Siegel, chirality cannot be transferred or forgotten and remembered.
9
All of the processes discussed in this paper
are rooted in structure and bonding (stereogenicity) not symmetry
(chirality). It is hoped that the readers of this paper will show
similar restraint.
Related collections
Most cited references6
- Record: found
- Abstract: not found
- Article: not found
A macromolecular conformational change driven by a minute chiral solvation energy
Norman C Peterson, Mark M. Green, Chetan Khatri (1993)
- Record: found
- Abstract: found
- Article: not found
Asymmetric Catalysis in Chiral Solvents: Chirality Transfer with Amplification of Homochirality through a Helical Macromolecular Scaffold
Yuuya Nagata, Ryohei Takeda, Michinori Suginome (2019)
- Record: found
- Abstract: not found
- Article: not found
Poly(quinoxaline-2,3-diyl)s: A Fascinating Helical Macromolecular Scaffold for New Chiral Functions
Michinori Suginome, Takeshi Yamamoto, Yuuya Nagata … (2015)
