- Record: found
- Abstract: found
- Article: found
Vangl2, a planar cell polarity molecule, is implicated in irreversible and reversible kidney glomerular injury
Abstract
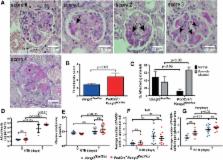
Planar cell polarity (PCP) pathways control the orientation and alignment of epithelial cells within tissues. Van Gogh‐like 2 (Vangl2) is a key PCP protein that is required for the normal differentiation of kidney glomeruli and tubules. Vangl2 has also been implicated in modifying the course of acquired glomerular disease, and here, we further explored how Vangl2 impacts on glomerular pathobiology in this context. Targeted genetic deletion of Vangl2 in mouse glomerular epithelial podocytes enhanced the severity of not only irreversible accelerated nephrotoxic nephritis but also lipopolysaccharide‐induced reversible glomerular damage. In each proteinuric model, genetic deletion of Vangl2 in podocytes was associated with an increased ratio of active‐MMP9 to inactive MMP9, an enzyme involved in tissue remodelling. In addition, by interrogating microarray data from two cohorts of renal patients, we report increased VANGL2 transcript levels in the glomeruli of individuals with focal segmental glomerulosclerosis, suggesting that the molecule may also be involved in certain human glomerular diseases. These observations support the conclusion that Vangl2 modulates glomerular injury, at least in part by acting as a brake on MMP9, a potentially harmful endogenous enzyme. © 2018 The Authors. The Journal of Pathology published by John Wiley & Sons Ltd on behalf of Pathological Society of Great Britain and Ireland.
Related collections
Most cited references61
- Record: found
- Abstract: found
- Article: not found
Matrix metalloproteinases and their inhibitors in connective tissue remodeling.
- Record: found
- Abstract: found
- Article: not found
Inflammatory macrophage migration requires MMP-9 activation by plasminogen in mice.
- Record: found
- Abstract: found
- Article: not found