- Record: found
- Abstract: found
- Article: found
Corticosteroid co-treatment induces resistance to chemotherapy in surgical resections, xenografts and established cell lines of pancreatic cancer
Read this article at
Abstract
Background
Chemotherapy for pancreatic carcinoma often has severe side effects that limit its efficacy. The glucocorticoid (GC) dexamethasone (DEX) is frequently used as co-treatment to prevent side effects of chemotherapy such as nausea, for palliative purposes and to treat allergic reactions. While the potent pro-apoptotic properties and the supportive effects of GCs to tumour therapy in lymphoid cells are well studied, the impact of GCs to cytotoxic treatment of pancreatic carcinoma is unknown.
Methods
A prospective study of DEX-mediated resistance was performed using a pancreatic carcinoma xenografted to nude mice, 20 surgical resections and 10 established pancreatic carcinoma cell lines. Anti-apoptotic signaling in response to DEX was examined by Western blot analysis.
Results
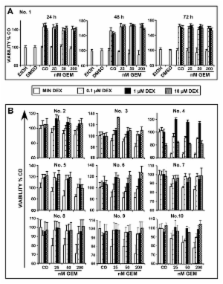
In vitro, DEX inhibited drug-induced apoptosis and promoted the growth in all of 10 examined malignant cells. Ex vivo, DEX used in physiological concentrations significantly prevented the cytotoxic effect of gemcitabine and cisplatin in 18 of 20 freshly isolated cell lines from resected pancreatic tumours. No correlation with age, gender, histology, TNM and induction of therapy resistance by DEX co-treatment could be detected. In vivo, DEX totally prevented cytotoxicity of chemotherapy to pancreatic carcinoma cells xenografted to nude mice. Mechanistically, DEX upregulated pro-survival factors and anti-apoptotic genes in established pancreatic carcinoma cells.
Related collections
Most cited references30
- Record: found
- Abstract: found
- Article: not found
Adjuvant chemoradiotherapy and chemotherapy in resectable pancreatic cancer: a randomised controlled trial.
- Record: found
- Abstract: found
- Article: not found
Microarray analysis reveals glucocorticoid-regulated survival genes that are associated with inhibition of apoptosis in breast epithelial cells.
- Record: found
- Abstract: found
- Article: not found