- Record: found
- Abstract: found
- Article: found
Changing muscle function with sustained glial derived neurotrophic factor treatment of rabbit extraocular muscle

Read this article at
Abstract
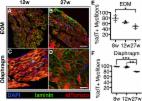
Recent microarray and RNAseq experiments provided evidence that glial derived neurotrophic factor (GDNF) levels were decreased in extraocular muscles from human strabismic subjects compared to age-matched controls. We assessed the effect of sustained GDNF treatment of the superior rectus muscles of rabbits on their physiological and morphological characteristics, and these were compared to naïve control muscles. Superior rectus muscles of rabbits were implanted with a sustained release pellet of GDNF to deliver 2μg/day, with the contralateral side receiving a placebo pellet. After one month, the muscles were assessed using in vitro physiological methods. The muscles were examined histologically for alteration in fiber size, myosin expression patterns, neuromuscular junction size, and stem cell numbers and compared to age-matched naïve control muscles. GDNF resulted in decreased force generation, which was also seen on the untreated contralateral superior rectus muscles. Muscle relaxation times were increased in the GDNF treated muscles. Myofiber mean cross-sectional areas were increased after the GDNF treatment, but there was a compensatory increase in expression of developmental, neonatal, and slow tonic myosin heavy chain isoforms. In addition, in the GDNF treated muscles there was a large increase in Pitx2-positive myogenic precursor cells. One month of GDNF resulted in significant extraocular muscle adaptation. These changes are interesting relative to the decreased levels of GDNF in the muscles from subjects with strabismus and preliminary data in infant non-human primates where sustained GDNF treatment produced a strabismus. These data support the view that GDNF has the potential for improving eye alignment in subjects with strabismus.
Related collections
Most cited references47
- Record: found
- Abstract: found
- Article: found
Pervasive satellite cell contribution to uninjured adult muscle fibers

- Record: found
- Abstract: found
- Article: found
Treadmill Exercise Induced Functional Recovery after Peripheral Nerve Repair Is Associated with Increased Levels of Neurotrophic Factors
- Record: found
- Abstract: found
- Article: not found