- Record: found
- Abstract: found
- Article: found
Response to First-Line Ritonavir-Boosted Protease Inhibitors (PI/r)-Based Regimens in HIV Positive Patients Presenting to Care with Low CD4 Counts: Data from the Icona Foundation Cohort
Abstract
Background
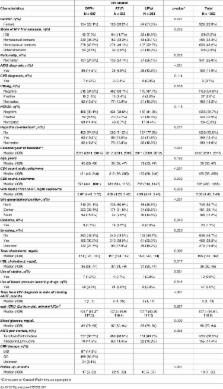
There are no data comparing the response to PI/r-based regimens in people presenting for care with low CD4 counts or AIDS (LC).
Aim
To compare the response to LPV/r-, DRV/r- or ATV/r-based cART regimens in LC initiating cART from ART-naive.
Methods
We included people enrolled in Icona with either CD4 counts ≤350 cells/mm 3 (low CD4-LC) or CD4 counts ≤200 cells/mm 3 (very low CD4-VLC) and/or AIDS, starting their first PI/r-based regimen after 2008. Initial regimens were compared by intention-to-treat: i) time to viral failure (VF) (first of 2 consecutive VL>200 copies/mL after≥6 months); II) time to PI/r discontinuation/switching for any cause (TD) and for toxicity (TDT); III) treatment failure (TF) (VF or TD). Kaplan-Meier and Cox analyses were used.
Results
1,362 LC patients were included (DRV/r 607; ATV/r 552; LPV/r 203); 813 VLC. In a median of 18 months (IQR:7–35), the 1-year probability of VF and TF were 2.8% (1.9–3.8) and 21.1% (18.7–23.4). In the adjusted analysis, patients initiating ATV/r had a 53% lower chance, and those initiating DRV/r a 61% lower chance of TD, as compared to LPV/r; the risk of TF was more likely in people starting LPV/r. Results were similar among VLC; in this subgroup LPV/r including regimens demonstrated a lower chance of VF.
Related collections
Most cited references14
- Record: found
- Abstract: found
- Article: found
Initiation of Antiretroviral Therapy in Early Asymptomatic HIV Infection
- Record: found
- Abstract: found
- Article: not found
Once-daily dolutegravir versus darunavir plus ritonavir in antiretroviral-naive adults with HIV-1 infection (FLAMINGO): 48 week results from the randomised open-label phase 3b study.
- Record: found
- Abstract: found
- Article: not found