- Record: found
- Abstract: found
- Article: found
Ret inhibition decreases growth and metastatic potential of estrogen receptor positive breast cancer cells
Read this article at
Abstract
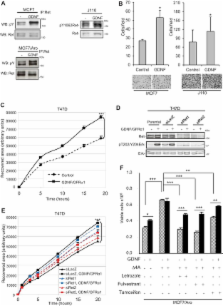
We show that elevated levels of Ret receptor are found in different sub-types of human breast cancers and that high Ret correlates with decreased metastasis-free survival. The role of Ret in ER+ breast cancer models was explored combining in vitro and in vivo approaches. Our analyses revealed that ligand-induced Ret activation: (i) stimulates migration of breast cancer cells; (ii) rescues cells from anti-proliferative effects of endocrine treatment and (iii) stimulates expression of cytokines in the presence of endocrine agents. Indeed, we uncovered a positive feed-forward loop between the inflammatory cytokine IL6 and Ret that links them at the expression and the functional level. In vivo inhibition of Ret in a metastatic breast cancer model inhibits tumour outgrowth and metastatic potential. Ret inhibition blocks the feed-forward loop by down-regulating Ret levels, as well as decreasing activity of Fak, an integrator of IL6-Ret signalling. Our results suggest that Ret kinase should be considered as a novel therapeutic target in subsets of breast cancer.
Related collections
Most cited references33
- Record: found
- Abstract: found
- Article: not found
RET, ROS1 and ALK fusions in lung cancer.
- Record: found
- Abstract: found
- Article: not found
Diverse somatic mutation patterns and pathway alterations in human cancers.
- Record: found
- Abstract: found
- Article: not found