- Record: found
- Abstract: found
- Article: found
Large Extracellular Vesicle Characterization and Association with Circulating Tumor Cells in Metastatic Castrate Resistant Prostate Cancer
Read this article at
Abstract
Simple Summary
Non-invasive, liquid biopsies are an attractive means for tumor diagnosis and monitoring. In addition to DNA and cells, tumors have an increased propensity compared to normal cells to shed vesicles. These large extracellular vesicles, which are believed to be more frequent in aggressive cancers, carry tumor DNA and proteins and can thus be informative sources for diagnosis and prognosis. In this study, we developed a method to identify and molecularly characterize large extracellular vesicles in parallel to circulating tumor cells at a single-cell/single-vesicle level. We show that the number of large extracellular vesicles correlates to and exceeds the number of circulating tumor cells, and that analysis of tumor-derived large extracellular vesicles hence increases the sensitivity of the liquid biopsy assay.
Abstract
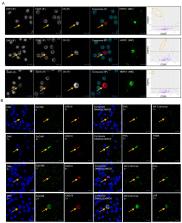
Liquid biopsies hold potential as minimally invasive sources of tumor biomarkers for diagnosis, prognosis, therapy prediction or disease monitoring. We present an approach for parallel single-object identification of circulating tumor cells (CTCs) and tumor-derived large extracellular vesicles (LEVs) based on automated high-resolution immunofluorescence followed by downstream multiplexed protein profiling. Identification of LEVs >6 µm in size and CTC enumeration was highly correlated, with LEVs being 1.9 times as frequent as CTCs, and additional LEVs were identified in 73% of CTC-negative liquid biopsy samples from metastatic castrate resistant prostate cancer. Imaging mass cytometry (IMC) revealed that 49% of cytokeratin (CK)-positive LEVs and CTCs were EpCAM-negative, while frequently carrying prostate cancer tumor markers including AR, PSA, and PSMA. HSPD1 was shown to be a specific biomarker for tumor derived circulating cells and LEVs. CTCs and LEVs could be discriminated based on size, morphology, DNA load and protein score but not by protein signatures. Protein profiles were overall heterogeneous, and clusters could be identified across object classes. Parallel analysis of CTCs and LEVs confers increased sensitivity for liquid biopsies and expanded specificity with downstream characterization. Combined, it raises the possibility of a more comprehensive assessment of the disease state for precise diagnosis and monitoring.
Related collections
Most cited references43
- Record: found
- Abstract: found
- Article: not found
Tumour exosome integrins determine organotropic metastasis
- Record: found
- Abstract: found
- Article: not found
Proteomic comparison defines novel markers to characterize heterogeneous populations of extracellular vesicle subtypes.
- Record: found
- Abstract: found
- Article: not found